France: Criminal Complaint on "Vaccines" (= gene therapy)
Association Réaction 19 16.12.2020
Translated by Claire Edwards
OFFICE OF PUBLIC PROSECUTION
OF THE FRENCH REPUBLIC
AT THE COURT OF PARIS
COMPLAlNT RELATING TO VACCINES
ARTICLE 40 OF THE CODE OF CRIMINAL PROCEDURE
FOR:[[1]].[[2]]
RÉACTION 19, Association governed by the law of 1901, registered at the Prefecture under number W751256495, domiciled at 63 rue la Boétie 75008, Paris and headed by Messrs. Carlo Alberto Brusa and Riccardo Mereu.
AGAINST :
X, any named person revealed as a result of the investigation
The facts of:
- The offence of deliberately endangering other persons
Article 223-1 of the Criminal Code
- The offence of aggravated deception
Articles L213-1 and L213-2 of the Consumer Code
- The offence of abuse of a person’s weakness
Article 223-15-2 of the Criminal Code
- The offence of aggravated extortion
Article 312-2 of the Criminal Code
HAS THE HONOUR TO PRESENT THE FACTS
* * *
Complaint Relating to Vaccines: Plan
l – SUMMARY OF FACTS AND PROCEDURE:
1. Health and political context
2. Medical controversy regarding the availability of a vaccine
3. Implementation of an novel gene therapy
4. Dangers to humans of a novel gene therapy
a) Secondary effects involving even the death of persons
b) The establishment of a derogation procedure permitting the dissemination of vaccines without issuance of a marketing authorisation and without evaluation by the scientific community
c) Knowledge of the risks and damages expected by the authorities: the pharmaceutical laboratories and the medical profession and their [vaccine] management organised in advance
5. Violation of international and constitutional instruments
a) Violation of international instruments
b) Violation of the precautionary principle
II – ACTS COMMITTED CAUSING DAMAGE TO THE PERSONS REPRESENTED BY THE REACTION 19 ASSOCIATION CONSTITUTE SERIOUS CRIMINAL OFFENCES
1. The offence of deliberately endangering the life of other persons
a) Existence of a special duty of care or prudence under the law or regulations
b) Wilful violation of special duty of prudence under the law or regulations
c) Existence of immediate risk of death or serious injury
2. The offence of deception
a) Substance of the offence of deception
b) Intentional element of the offence of deception
3. The offence of fraudulent abuse of the state of ignorance or weakness
a) Preconditions of the offence of fraudulent abuse of the state of ignorance or weakness
b) Substantive element [actus reus] of the offence of fraudulent abuse of the state of ignorance or weakness
c) Mental element [mens rea] of the offence of abuse of weakness
4. The offence of extortion
a) Substance [actus reus] of the offence of extortion
b) The intentional element of the offence of extortion
I – SUMMARY OF FACTS AND PROCEDURE:
1. Health and political context:
Since the beginning of the health crisis linked to the viral disease Covid-19, the “vaccine” has been designated as the sole solution to definitively bring to an end the Covid-19 pandemic, the origin of which still remains unknown.
As early as March 2020, the [pharmaceutical] laboratories undertook to provide a “vaccine” against Covid-19 in the coming 12 to 18 months, despite the “development of a vaccine usually requiring from 10 to 15 years”.
In mid-November, several pharmaceutical laboratories issued press releases on the first results on efficacy.
The Pfizer, BioNTech then Moderna laboratories declared one after the other that they had created a “vaccine” against Covid-19 that was more than 90%, then 95% effective.
All of these studies were carried out without any transparency, in worryingly record time, and without allowing an independent body to verify their results, even minimally.
Professor Christian Perronne warned about this in a statement published by France Soir on 8 December 2020:
“The most worrying thing is that numerous countries, including France, say that they are ready to vaccinate in the weeks to come, while the finalisation and evaluation of these products are being rushed and not a single result on the efficacy or the hazardousness of these vaccines has been published to date. We were only allowed to see industry and manufacturers’ press releases, which enabled them to drive up their share prices on the stock markets.” Exhibit No. 1
Indeed, it is proved that there is no certainty with regard to the efficacy of this “vaccine”.
This is evidenced by Alain Fischer himself, an immunologist appointed by the Prime Minister to coordinate the State’s vaccine strategy against Covid-19, stating on 5 December 2020:
“The solution will take time, to know if the vaccine, on the one hand, protects the vaccinated individual against infection … but also protects against transmission … it will probably take several months before we have this type of information which will have an impact on vaccination policies” (emphasis added) Exhibit No. 2
Thus the person in charge of vaccination in France explains clearly that on 5 December and for several months to come it will be impossible to know the efficacy of the “vaccine” proposed by the various laboratories.
It is even more worrying that the Pfizer pharmaceutical group has already been the subject of a complaint in the United States for “fraudulent commercial practices” in the matter of the marketing of several drugs (Bextra, Zyvox, Geodon and Lyrica) and was forced to pay a “record” fine of 2.3 billion dollars. Exhibit No. 5
Furthermore, the clinical trials have revealed the side effects identified after receiving the Pfizer vaccine against Covid-19:
“After receiving the injection, 63% of the trial participants reported tiredness and 55% stated that they had headaches. Chills were reported by 32% of the participants, 24% complained of joint pain and 14% developed a fever.” Exhibit No. 3
Even more serious, some patients suffered Bell’s palsy, a disorder of the facial nerves that involves paralysis of the face, and six of them died during the clinical trials. Exhibit No. 4
Yet it is in this context of risks and total uncertainty that the President of the Republic asserted, in his speech of 24 November 2020, in clear violation of the precautionary principle, that the “vaccination campaign” would start “at the end of December, beginning of January”.
Moreover, this announcement was made while the expediency of even the principle of vaccination in the framework of the Covid-19 virus is very controversial among medical professionals, in particular in view of its limited efficacy, its hazardousness and the shortness of time available to appraise this new technology.
2. Medical controversy surrounding even the expediency of a vaccine
According to Imperial College, London, after analysis of 175 studies published throughout the world, the real fatality rate of Covid-19, i.e. the percentage of deaths relative to the number of people infected, is estimated to be 1.15%, meaning that it is virtually non-existent! Exhibit No. 9
In addition, it has been revealed that the median age of those deceased from Covid-19 is 84 and that 90.8% of deaths were of people aged over 65. Exhibit No. 28
It is therefore old people who are the most at risk and who should be the focus of this “vaccination” plan.
Yet on 9 July 2020 the Scientific Committee delivered a note on the vaccination strategy, declaring in its highlights:
“In any case the question arises of immunising subjects aged over 75 in whom probably only a low vaccination response will be obtained and whom it will be necessary to cover by barrier-protection measures.” Exhibit No. 27
In other words, the “vaccine” tests do not immunise, or immunise minimally, the people at risk!
Furthermore, some scientists say that most of the population is already immunised against the virus.
Indeed, many scientists argue that cross-immunity, allowing immunity to Covid-19 to be achieved without ever having contracted it simply by having been in contact with other coronaviruses, is very likely.
Professor Didier RAOULT states in this respect:
“If you look at people who have had an infection, a significant number of them already have antibodies. So they cannot be infected by the coronavirus because they had an immunity before this epidemic … between 40 and 70% of the population were already immunised.”[iii] Exhibit No. 10
If between 40 and 70% of the population were already immunised before the epidemic, this proportion will have necessarily increased since the beginning of the epidemic.
Others argue that a vaccine alone will not be able to bring an end to the Covid-19 epidemic:
"A vaccine alone may not allow everything to return to normal unless both vaccine efficacy and vaccination coverage are fairly high [and] would require a potentially unachievable 100% coverage of the population."2 Exhibit No. 11
Finally, a recent poll carried out by BFMTV and published on 9 December 2020 reveals that 52% of French people state that they will not get vaccinated while only 32% state their willingness to be vaccinated. Exhibit No. 12
Thus, beyond the question of the health expediency of such a “vaccine” being introduced and of its efficacy, only a minority of French people wish to get vaccinated, so such a “vaccine” will not bring the Covid-19 epidemic to an end.
Moreover, it has to be specified that this much-discussed “vaccine”, so lauded by the Government and the pharmaceutical companies, is in reality a novel gene therapy.
3. The introduction of a novel gene therapy
The term “vaccine” used by the pharmaceutical laboratories and the members of the Government is a misuse of terminology.
In fact, what the laboratories are offering is in reality a gene therapy.
It is acknowledged that vaccination:
“[…] has the objective of stimulating the immune defences of a human or of an animal in regard to an infectious agent by exposing him or her voluntarily to this agent (in an attenuated or inactivated form) or to one of its components called antibody (generally a protein)” Exhibit No. 6
Whereas the “vaccines” offered by the Pfizer, BioNTech and Moderna laboratories involve:
“introducing viral genetic material into the cells of the person to be vaccinated (administration is mainly intramuscular, or intradermic in two of the cases). It is either RNA trapped in lipid nanoparticles, DNA inserted into a plasmid, or DNA or RNA delivered by an inactivated genetically modified virus.” Exhibit No. 6
This is why Dr. Christian Perronne, Head of the Infectious and Tropical Diseases Department at Garches Hospital, rejects the use of the term “vaccine” and states that:
“The first “vaccines” that we are being offered are not vaccines, but gene therapy products. They are going to inject nucleic acids that will cause our own cells to make elements of the virus.” Exhibit No. 1
A member of the European Parliament states in this respect:
“The first thing we need to understand is that these Covid-19 GMO vaccines are highly experimental drugs. We know almost nothing about their medium- to long-term genetic effects.
First of all, since 2003 and the surge of SARS in Asia, the scientific community has never managed to develop an anti-coronavirus vaccine. Secondly, there are several different GMO technologies used to develop the various anti-Covid-19 GMO vaccines being evaluated. Three of these GMO technologies have never been authorised for medicinal products for human use.” Exhibit No. 15
Thus, prior to the outbreak of Covid-19, no gene therapy product had been approved for humans. Exhibit No. 8
The proposed vaccines are therefore experimental, on the one hand because they have never been tested on human beings for treating a virus, and on the other hand because their function, originally curative, is henceforth preventive.
The report published in September 2020 by CRIIGEN [Committee for Independent Research and Information on Genetic Engineering] specifies in this respect:
“Gene therapy or immunotherapy involves not only a limited number of people but also seriously ill people. Consequently, not only do the possible side effects affect a small number of individuals, but the severity of their state of health and their health emergency doubtless causes them to accept some risk-taking. In the case of vaccines, we are in a preventive approach. This therefore concerns a large number of people, the vast majority of whom are in good health (at least in terms of the disease that the vaccine is supposed to protect us from).” Exhibit No. 6
Vaccination is therefore a preventive method, used to avoid contracting the disease, whereas gene therapy is a curative method, used to treat a person who has already contracted the disease.
Gene therapies are therefore generally reserved for sick people, especially those suffering from serious diseases in view of the possible risks involved.
Thus, using gene therapy to carry out a "mass vaccination plan" amounts to taking reckless risks with healthy people whose contamination by the virus would pose little danger (for those under 65 years of age without comorbidities).
Moreover, this therapy has never been used on humans before to fight a virus. No consideration has been given to analysing either its efficacy or, even more importantly, its adverse effects on health.
4. Dangers to humans of a novel gene therapy
a) Side effects may include death
Thus, many scientists warn about the serious side effects that would result from the use of such gene therapy products.
Dr. Hugues TOLOU, an expert working for Public Health Belgium, the National Authority for Health (HAS) and the European Centre for Disease Prevention and Control (ECDC), points out in this respect that:
“It is still too early to confirm the safety of the vaccines for the general population:
· RNA vaccines cause the cells of vaccinated people to produce antigens. These cells thus become the target of the immune response, as is the case during a viral infection. This normal process can be the cause of undesirable effects if it is too intense or extensive, or if it affects non-replaceable cells. RNA not incorporated by cells could furthermore have toxic effects.
In the case of Covid-19, is it possible that the immunity developed after infection or through vaccination can play a harmful role? There has been much talk of a runaway immune response or "cytokine storm" that can worsen the course of infection in some patients and justify the trial of anti-inflammatory and immunosuppressive treatments. There is also concern about the possibility that some antibodies unable to neutralize the virus act instead as facilitators of infection, by a mechanism called ADE (Antibody-dependent enhancement).” Exhibit No. 34
Geneticist Alexandra HENRION-CAUDE supports this analysis by stating:
"[There is] a risk of developing a runaway immune response in terms of antibody production.” [iv] Exhibit No. 33
Gene therapies can also be the cause of cancer development.
Discussing this aspect, an MEP stated on 7 September 2020, about a trial led by Alain Fischer:
"Let’s not forget that a gene therapy trial using a vector virus, an adenovirus similar to the anti-Covid-19 candidate vaccine by AstraZeneca, led to the appearance of blood cancer in two of the ten bubble babies [bubble baby disease or Severe Combined Immunodeficiency Disorder (SCID)] participating in the trial supervised by immunology professor Alain Fischer in 2003. Specialists speak of ‘insertional oncogenesis’ to describe this risk of cancer induced by genetic manipulation.” Exhibit No. 15
The gene therapy trial conducted by Alain Fischer resulted in 20% of the subjects developing blood cancer. It is therefore not surprising that he now calls for caution regarding this practice.
Furthermore, a report by the Committee for Independent Research and Information on Genetic Engineering (CRIIGEN) published in September 2020 clearly sets out the risks of vaccines delivering RNA or DNA encoding the protein antigen:
“3.1. The risk of the occurrence of recombinant viruses
This risk is independent of the vector used to deliver the viral DNA or RNA encoding the protein antigen to host cells, whether a plasmid vector, a nanoparticle, or a genetically modified virus. However, this risk is even greater when genetically modified viruses are used because they bring not only the DNA or viral RNA of interest but also part of their own genome.
[1] [Translation by Claire Edwards, BA Hons, MA, United Nations Editor (Retired). Translator’s explanatory notes appear between square brackets.]
[2] [Article 40: “Le procureur de la République reçoit les plaintes et les dénonciations et apprécie la suite à leur donner conformément aux dispositions de l'article 40-1. Toute autorité constituée, tout officier public ou fonctionnaire qui, dans l'exercice de ses fonctions, acquiert la connaissance d'un crime ou d'un délit est tenu d'en donner avis sans délai au procureur de la République et de transmettre à ce magistrat tous les renseignements, procès-verbaux et actes qui y sont relatifs.” (“The district prosecutor receives complaints and denunciations and decides how to deal with them, in accordance with the provisions of article 40-1. Every constituted authority, every public officer or civil servant who, in the performance of his duties, has gained knowledge of the existence of a felony or of a misdemeanour is obliged to notify forthwith the district prosecutor of the offence and to transmit to this prosecutor any relevant information, official reports or documents.”)]
[iii] Prof. Didier RAOULT. Youtube interview: https://youtu.be/2UbithnaK0?t=568.
[iv] Sud Radio interview, 16 November 2020, https://www.sudradio.fr/societe/alexandra-henrion-caude-jai-limpression-quon-est-revenu-au-temps-des-devins/.
OF THE FRENCH REPUBLIC
AT THE COURT OF PARIS
COMPLAlNT RELATING TO VACCINES
ARTICLE 40 OF THE CODE OF CRIMINAL PROCEDURE
FOR:[[1]].[[2]]
RÉACTION 19, Association governed by the law of 1901, registered at the Prefecture under number W751256495, domiciled at 63 rue la Boétie 75008, Paris and headed by Messrs. Carlo Alberto Brusa and Riccardo Mereu.
AGAINST :
X, any named person revealed as a result of the investigation
The facts of:
- The offence of deliberately endangering other persons
Article 223-1 of the Criminal Code
- The offence of aggravated deception
Articles L213-1 and L213-2 of the Consumer Code
- The offence of abuse of a person’s weakness
Article 223-15-2 of the Criminal Code
- The offence of aggravated extortion
Article 312-2 of the Criminal Code
HAS THE HONOUR TO PRESENT THE FACTS
* * *
Complaint Relating to Vaccines: Plan
l – SUMMARY OF FACTS AND PROCEDURE:
1. Health and political context
2. Medical controversy regarding the availability of a vaccine
3. Implementation of an novel gene therapy
4. Dangers to humans of a novel gene therapy
a) Secondary effects involving even the death of persons
b) The establishment of a derogation procedure permitting the dissemination of vaccines without issuance of a marketing authorisation and without evaluation by the scientific community
c) Knowledge of the risks and damages expected by the authorities: the pharmaceutical laboratories and the medical profession and their [vaccine] management organised in advance
5. Violation of international and constitutional instruments
a) Violation of international instruments
b) Violation of the precautionary principle
II – ACTS COMMITTED CAUSING DAMAGE TO THE PERSONS REPRESENTED BY THE REACTION 19 ASSOCIATION CONSTITUTE SERIOUS CRIMINAL OFFENCES
1. The offence of deliberately endangering the life of other persons
a) Existence of a special duty of care or prudence under the law or regulations
b) Wilful violation of special duty of prudence under the law or regulations
c) Existence of immediate risk of death or serious injury
2. The offence of deception
a) Substance of the offence of deception
b) Intentional element of the offence of deception
3. The offence of fraudulent abuse of the state of ignorance or weakness
a) Preconditions of the offence of fraudulent abuse of the state of ignorance or weakness
b) Substantive element [actus reus] of the offence of fraudulent abuse of the state of ignorance or weakness
c) Mental element [mens rea] of the offence of abuse of weakness
4. The offence of extortion
a) Substance [actus reus] of the offence of extortion
b) The intentional element of the offence of extortion
I – SUMMARY OF FACTS AND PROCEDURE:
1. Health and political context:
Since the beginning of the health crisis linked to the viral disease Covid-19, the “vaccine” has been designated as the sole solution to definitively bring to an end the Covid-19 pandemic, the origin of which still remains unknown.
As early as March 2020, the [pharmaceutical] laboratories undertook to provide a “vaccine” against Covid-19 in the coming 12 to 18 months, despite the “development of a vaccine usually requiring from 10 to 15 years”.
In mid-November, several pharmaceutical laboratories issued press releases on the first results on efficacy.
The Pfizer, BioNTech then Moderna laboratories declared one after the other that they had created a “vaccine” against Covid-19 that was more than 90%, then 95% effective.
All of these studies were carried out without any transparency, in worryingly record time, and without allowing an independent body to verify their results, even minimally.
Professor Christian Perronne warned about this in a statement published by France Soir on 8 December 2020:
“The most worrying thing is that numerous countries, including France, say that they are ready to vaccinate in the weeks to come, while the finalisation and evaluation of these products are being rushed and not a single result on the efficacy or the hazardousness of these vaccines has been published to date. We were only allowed to see industry and manufacturers’ press releases, which enabled them to drive up their share prices on the stock markets.” Exhibit No. 1
Indeed, it is proved that there is no certainty with regard to the efficacy of this “vaccine”.
This is evidenced by Alain Fischer himself, an immunologist appointed by the Prime Minister to coordinate the State’s vaccine strategy against Covid-19, stating on 5 December 2020:
“The solution will take time, to know if the vaccine, on the one hand, protects the vaccinated individual against infection … but also protects against transmission … it will probably take several months before we have this type of information which will have an impact on vaccination policies” (emphasis added) Exhibit No. 2
Thus the person in charge of vaccination in France explains clearly that on 5 December and for several months to come it will be impossible to know the efficacy of the “vaccine” proposed by the various laboratories.
It is even more worrying that the Pfizer pharmaceutical group has already been the subject of a complaint in the United States for “fraudulent commercial practices” in the matter of the marketing of several drugs (Bextra, Zyvox, Geodon and Lyrica) and was forced to pay a “record” fine of 2.3 billion dollars. Exhibit No. 5
Furthermore, the clinical trials have revealed the side effects identified after receiving the Pfizer vaccine against Covid-19:
“After receiving the injection, 63% of the trial participants reported tiredness and 55% stated that they had headaches. Chills were reported by 32% of the participants, 24% complained of joint pain and 14% developed a fever.” Exhibit No. 3
Even more serious, some patients suffered Bell’s palsy, a disorder of the facial nerves that involves paralysis of the face, and six of them died during the clinical trials. Exhibit No. 4
Yet it is in this context of risks and total uncertainty that the President of the Republic asserted, in his speech of 24 November 2020, in clear violation of the precautionary principle, that the “vaccination campaign” would start “at the end of December, beginning of January”.
Moreover, this announcement was made while the expediency of even the principle of vaccination in the framework of the Covid-19 virus is very controversial among medical professionals, in particular in view of its limited efficacy, its hazardousness and the shortness of time available to appraise this new technology.
2. Medical controversy surrounding even the expediency of a vaccine
According to Imperial College, London, after analysis of 175 studies published throughout the world, the real fatality rate of Covid-19, i.e. the percentage of deaths relative to the number of people infected, is estimated to be 1.15%, meaning that it is virtually non-existent! Exhibit No. 9
In addition, it has been revealed that the median age of those deceased from Covid-19 is 84 and that 90.8% of deaths were of people aged over 65. Exhibit No. 28
It is therefore old people who are the most at risk and who should be the focus of this “vaccination” plan.
Yet on 9 July 2020 the Scientific Committee delivered a note on the vaccination strategy, declaring in its highlights:
“In any case the question arises of immunising subjects aged over 75 in whom probably only a low vaccination response will be obtained and whom it will be necessary to cover by barrier-protection measures.” Exhibit No. 27
In other words, the “vaccine” tests do not immunise, or immunise minimally, the people at risk!
Furthermore, some scientists say that most of the population is already immunised against the virus.
Indeed, many scientists argue that cross-immunity, allowing immunity to Covid-19 to be achieved without ever having contracted it simply by having been in contact with other coronaviruses, is very likely.
Professor Didier RAOULT states in this respect:
“If you look at people who have had an infection, a significant number of them already have antibodies. So they cannot be infected by the coronavirus because they had an immunity before this epidemic … between 40 and 70% of the population were already immunised.”[iii] Exhibit No. 10
If between 40 and 70% of the population were already immunised before the epidemic, this proportion will have necessarily increased since the beginning of the epidemic.
Others argue that a vaccine alone will not be able to bring an end to the Covid-19 epidemic:
"A vaccine alone may not allow everything to return to normal unless both vaccine efficacy and vaccination coverage are fairly high [and] would require a potentially unachievable 100% coverage of the population."2 Exhibit No. 11
Finally, a recent poll carried out by BFMTV and published on 9 December 2020 reveals that 52% of French people state that they will not get vaccinated while only 32% state their willingness to be vaccinated. Exhibit No. 12
Thus, beyond the question of the health expediency of such a “vaccine” being introduced and of its efficacy, only a minority of French people wish to get vaccinated, so such a “vaccine” will not bring the Covid-19 epidemic to an end.
Moreover, it has to be specified that this much-discussed “vaccine”, so lauded by the Government and the pharmaceutical companies, is in reality a novel gene therapy.
3. The introduction of a novel gene therapy
The term “vaccine” used by the pharmaceutical laboratories and the members of the Government is a misuse of terminology.
In fact, what the laboratories are offering is in reality a gene therapy.
It is acknowledged that vaccination:
“[…] has the objective of stimulating the immune defences of a human or of an animal in regard to an infectious agent by exposing him or her voluntarily to this agent (in an attenuated or inactivated form) or to one of its components called antibody (generally a protein)” Exhibit No. 6
Whereas the “vaccines” offered by the Pfizer, BioNTech and Moderna laboratories involve:
“introducing viral genetic material into the cells of the person to be vaccinated (administration is mainly intramuscular, or intradermic in two of the cases). It is either RNA trapped in lipid nanoparticles, DNA inserted into a plasmid, or DNA or RNA delivered by an inactivated genetically modified virus.” Exhibit No. 6
This is why Dr. Christian Perronne, Head of the Infectious and Tropical Diseases Department at Garches Hospital, rejects the use of the term “vaccine” and states that:
“The first “vaccines” that we are being offered are not vaccines, but gene therapy products. They are going to inject nucleic acids that will cause our own cells to make elements of the virus.” Exhibit No. 1
A member of the European Parliament states in this respect:
“The first thing we need to understand is that these Covid-19 GMO vaccines are highly experimental drugs. We know almost nothing about their medium- to long-term genetic effects.
First of all, since 2003 and the surge of SARS in Asia, the scientific community has never managed to develop an anti-coronavirus vaccine. Secondly, there are several different GMO technologies used to develop the various anti-Covid-19 GMO vaccines being evaluated. Three of these GMO technologies have never been authorised for medicinal products for human use.” Exhibit No. 15
Thus, prior to the outbreak of Covid-19, no gene therapy product had been approved for humans. Exhibit No. 8
The proposed vaccines are therefore experimental, on the one hand because they have never been tested on human beings for treating a virus, and on the other hand because their function, originally curative, is henceforth preventive.
The report published in September 2020 by CRIIGEN [Committee for Independent Research and Information on Genetic Engineering] specifies in this respect:
“Gene therapy or immunotherapy involves not only a limited number of people but also seriously ill people. Consequently, not only do the possible side effects affect a small number of individuals, but the severity of their state of health and their health emergency doubtless causes them to accept some risk-taking. In the case of vaccines, we are in a preventive approach. This therefore concerns a large number of people, the vast majority of whom are in good health (at least in terms of the disease that the vaccine is supposed to protect us from).” Exhibit No. 6
Vaccination is therefore a preventive method, used to avoid contracting the disease, whereas gene therapy is a curative method, used to treat a person who has already contracted the disease.
Gene therapies are therefore generally reserved for sick people, especially those suffering from serious diseases in view of the possible risks involved.
Thus, using gene therapy to carry out a "mass vaccination plan" amounts to taking reckless risks with healthy people whose contamination by the virus would pose little danger (for those under 65 years of age without comorbidities).
Moreover, this therapy has never been used on humans before to fight a virus. No consideration has been given to analysing either its efficacy or, even more importantly, its adverse effects on health.
4. Dangers to humans of a novel gene therapy
a) Side effects may include death
Thus, many scientists warn about the serious side effects that would result from the use of such gene therapy products.
Dr. Hugues TOLOU, an expert working for Public Health Belgium, the National Authority for Health (HAS) and the European Centre for Disease Prevention and Control (ECDC), points out in this respect that:
“It is still too early to confirm the safety of the vaccines for the general population:
· RNA vaccines cause the cells of vaccinated people to produce antigens. These cells thus become the target of the immune response, as is the case during a viral infection. This normal process can be the cause of undesirable effects if it is too intense or extensive, or if it affects non-replaceable cells. RNA not incorporated by cells could furthermore have toxic effects.
In the case of Covid-19, is it possible that the immunity developed after infection or through vaccination can play a harmful role? There has been much talk of a runaway immune response or "cytokine storm" that can worsen the course of infection in some patients and justify the trial of anti-inflammatory and immunosuppressive treatments. There is also concern about the possibility that some antibodies unable to neutralize the virus act instead as facilitators of infection, by a mechanism called ADE (Antibody-dependent enhancement).” Exhibit No. 34
Geneticist Alexandra HENRION-CAUDE supports this analysis by stating:
"[There is] a risk of developing a runaway immune response in terms of antibody production.” [iv] Exhibit No. 33
Gene therapies can also be the cause of cancer development.
Discussing this aspect, an MEP stated on 7 September 2020, about a trial led by Alain Fischer:
"Let’s not forget that a gene therapy trial using a vector virus, an adenovirus similar to the anti-Covid-19 candidate vaccine by AstraZeneca, led to the appearance of blood cancer in two of the ten bubble babies [bubble baby disease or Severe Combined Immunodeficiency Disorder (SCID)] participating in the trial supervised by immunology professor Alain Fischer in 2003. Specialists speak of ‘insertional oncogenesis’ to describe this risk of cancer induced by genetic manipulation.” Exhibit No. 15
The gene therapy trial conducted by Alain Fischer resulted in 20% of the subjects developing blood cancer. It is therefore not surprising that he now calls for caution regarding this practice.
Furthermore, a report by the Committee for Independent Research and Information on Genetic Engineering (CRIIGEN) published in September 2020 clearly sets out the risks of vaccines delivering RNA or DNA encoding the protein antigen:
“3.1. The risk of the occurrence of recombinant viruses
This risk is independent of the vector used to deliver the viral DNA or RNA encoding the protein antigen to host cells, whether a plasmid vector, a nanoparticle, or a genetically modified virus. However, this risk is even greater when genetically modified viruses are used because they bring not only the DNA or viral RNA of interest but also part of their own genome.
[1] [Translation by Claire Edwards, BA Hons, MA, United Nations Editor (Retired). Translator’s explanatory notes appear between square brackets.]
[2] [Article 40: “Le procureur de la République reçoit les plaintes et les dénonciations et apprécie la suite à leur donner conformément aux dispositions de l'article 40-1. Toute autorité constituée, tout officier public ou fonctionnaire qui, dans l'exercice de ses fonctions, acquiert la connaissance d'un crime ou d'un délit est tenu d'en donner avis sans délai au procureur de la République et de transmettre à ce magistrat tous les renseignements, procès-verbaux et actes qui y sont relatifs.” (“The district prosecutor receives complaints and denunciations and decides how to deal with them, in accordance with the provisions of article 40-1. Every constituted authority, every public officer or civil servant who, in the performance of his duties, has gained knowledge of the existence of a felony or of a misdemeanour is obliged to notify forthwith the district prosecutor of the offence and to transmit to this prosecutor any relevant information, official reports or documents.”)]
[iii] Prof. Didier RAOULT. Youtube interview: https://youtu.be/2UbithnaK0?t=568.
[iv] Sud Radio interview, 16 November 2020, https://www.sudradio.fr/societe/alexandra-henrion-caude-jai-limpression-quon-est-revenu-au-temps-des-devins/.
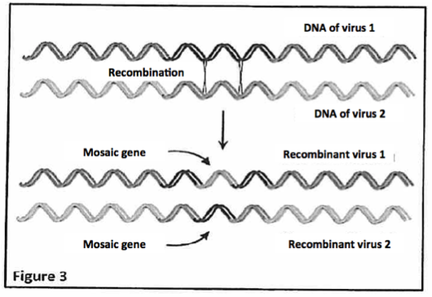
Viruses can easily exchange fragments of their respective genetic material when the viral genomes concerned are of the same kind (either DNA or RNA) and they share similar sequences (genes). The well-understood process that governs these exchanges is called recombination. When this recombination takes place between similar sequences of DNA or RNA, it is called homologous recombination. This phenomenon of recombination is not unique to viral DNA or RNA but viral sequences are known to be the subject of many recombinations. These are therefore described as "highly recombinogenic". This is why these recombinations between viral genetic materials of the so-called "recombinant" viruses whose gene or genes have been the site of these exchanges are called "mosaic", which means consisting partly of sequences from virus 1 and sequences from virus 2 (Figure 3). Figure 3 illustrates recombination between viral DNA but this phenomenon can also occur between viral RNA.
In some cases, these recombinant viruses are much more virulent than the original viruses and can therefore cause serious viral diseases. This phenomenon has been widely demonstrated in transgenic plants in which a viral gene has been deliberately introduced into their genome, and infected with a virus related to the one from which the viral transgene originates [8-16]. One high-profile example of a recombinant virus that can cause serious viral diseases in humans is that of the 2009 H1N1 virus, recombinant between three strains of influenza virus: a swine strain, a human strain and an avian strain [17, 18].
Of course, this phenomenon can only occur if genetic material from at least two viruses ends up in the same cells, which is fortunately extremely rare in nature since it implies that the same cells are co-infected with at least two viruses. But when viruses are man-made, this phenomenon can become much more common. This applies of course, as mentioned above, in the case of transgenic plants into which a viral transgene has been introduced when these plants need only be infected with a single virus for such recombination events to take place. But we run the same risk in humans when we generate vaccines introducing viral RNA or DNA into the cells of patients. Anti-Covid-19 vaccines of this type in clinical trials are administered intramuscularly or intradermally. The target cells are therefore muscle cells, skin cells and fibroblasts (cells of connective tissue, which means support tissue that envelops organs, tissues, and in particular muscular bundles) but also circulating blood cells and endothelial cells (which line the blood vessels), as well as many cells that can be the target of infections by other viruses. For example, enteroviruses (nonenveloped RNA viruses) have been detected in muscle cells [19], the Zika virus infects skin cells [20], the Chikungunya virus targets satellite muscle cells (muscle stem cells) [21], but also endothelial cells and fibroblasts [22]. And these are probably but a few examples ...
Vaccination against Covid-19, if it becomes a reality, will be a mass vaccination throughout the whole world. The probability of these kinds of events occurring is therefore far from being zero even if it is low in terms of frequency. Such mass vaccination with this type of vaccine could produce a huge number of new recombinant viruses. Let us not forget that it is enough for a single new virus to appear somewhere in the world for the health, environmental and social consequences to be global and vast ...
V.2. Genotoxicity: risk of insertional mutagenesis
Insertional mutagenesis is a mutation, in other words a modification of genetic information by inserting a sequence inside a genome, which can then inactivate or modify the expression of one or more genes.
This risk of genotoxicity for the human cells targeted by vaccination (whose genome is of course DNA) therefore concerns only vaccines delivering viral DNA, whether the vector is a plasmid or a genetically modified virus. However, this risk may also apply to vaccines delivering RNA through a genetically modified RNA viral vector such as the AIDS virus (HIV, widely used as a vector) if it has not been correctly stripped of its reverse transcriptase and the gene encoding it. Indeed, viral reverse transcriptase can then convert the delivered RNA into DNA, which will integrate into the genome of the target cells.
Genetically modified viruses are also widely used for gene therapy purposes to deliver in this case the normal version of a defective (mutated) human gene in the treated patient. In 2002, three years after a gene therapy trial (in children with severe immunodeficiency due to a mutation on an X chromosome gene) using a genetically modified RNA virus as a vector, two of the 10 children treated developed leukaemia due to the insertion of the repair DNA delivered by the viral vector near a proto-oncogene (cancer gene), causing a severe disruption in the expression of this [23]. Several studies have shown the effects of insertional mutagenesis caused by different families of RNA viruses {including HIV) [24]. Similarly, several studies in mice have shown that gene delivery by vectors derived from the adeno-associated virus (AAV, a small non-pathogenic DNA virus) results in insertional mutagenesis [25]. In 2016, a study on the genotoxic effects of viral vectors derived from HIV and AAV, used for gene therapy purposes, concluded that "a thorough understanding of viral biology and advances in cell genetics is needed in order to elucidate the nature of viral vector integration site selection and associated risks" [25 ].
V. lmmunotoxicity: risks specifically related to the use of modified viral vectors
In addition to the risks of occurrence of recombinant viruses and insertional mutagenesis, especially when the delivered genetic material is DNA, viral vectors can produce significant immunotoxicity effects given that they are themselves immunogenic. In 2002, a pilot gene therapy experiment carried out in 18 boys suffering from a severe metabolic disorder due to a failed gene located on the X chromosome led to the death of an 18-year-old man due to a fatal systemic inflammatory response caused by the viral vector (inactivated human DNA virus): DNA sequences of the vector were found in most of his tissues [27]. The fact that the other 17 persons treated did not show this type of response at all shows how difficult this risk is to predict and therefore difficult to overcome. In Belgium, several clinical trials of immunotherapy to fight cancer using an inactivated virus in which more than 15% of its genome was replaced by two human genes (encoding an antigen present on the surface of cancer cells and an interleukin, a protein that mediates communication between immune cells) showed a non-specific activation of the immune system linked to the vector resulting in an inflammatory reaction and an autoimmune response [28]. Many other studies have shown immunotoxicity effects of various viral vectors used for gene therapy or vaccination purposes [29-33]. In the case of viral vectors used for vaccination purposes, anti-vector immunity can also directly interfere with the desired vaccine efficacy (immunogenicity of the vaccine) [34].
V. General considerations for risk assessment of these vaccines
The use of vaccines that deliver viral genetic material (DNA or RNA) is new or recent. The use of genetically modified viruses as vectors, especially for gene therapy or immunotherapy, has shown the extent to which adverse reactions can be varied, uncontrolled and serious. Although immunotherapy efforts are relatively recent, we should not forget the failures of gene therapy over nearly 35 years.
These failures are largely explained by the race to the finish line at the expense of efficacy and/or biosecurity. Such an approach will never enable expectations or needs in terms of care to be met. (…)
Uncontrolled side effects would therefore have major repercussions, especially in a mass vaccination campaign such as the one to combat Covid-19. These repercussions could not only be disastrous in terms of health, of course, but also in terms of the environment, for example in the event of new recombinant viruses spreading. (See section IV.3.1.) And the fact that it is a preventive approach should not be a licence for risk-taking.
These vaccine candidates consequently require a thorough health and environmental assessment despite the urgency, whether arising from pressure from decision-making and health authorities or from the profit motive of the pharmaceutical industries embarked on this vaccine race. In its discussion paper of 23 July 2020 on the Covid-19 vaccine strategy [35], the National Authority for Health (HAS) stated: ‘In the context of the Covid-19 pandemic, the challenge is therefore to develop as effective and safe a vaccine as possible in record time.’ This exhortation on the part of an authority such as HAS is as nonsensical as it is outrageous.” Exhibit No. 1
This report makes edifying reading: the possible side effects and complications are very serious and may include death.
Yet despite the acknowledged dangers and side effects, the European Union has taken the liberty of removing the safeguards that it had itself imposed regarding the manipulation of genetically modified organisms (GMOs), as well as the requirements for environmental risk assessment and prior authorisation or consent specified by Directives 2009/41/EC and 2001/18/EC.
b) Establishment of a derogation procedure allowing the dissemination of vaccines without the issuance of a marketing authorisation and without evaluation by the scientific community
By Regulation 2020/1043 adopted by emergency procedure on 15 July 2020, in preambular paragraph 17, the European Union introduced a derogation regime for GMO manipulation and experimental medicinal products in these terms:
“(17) The main objective of Union legislation on medicinal products is to safeguard public health. That legislative framework is supplemented by the rules in Directive 2001/20/EC laying down specific standards for the protection of clinical trial subjects. Directives 2001/18/EC and 2009/41/EC have as their objective to ensure a high level of protection of human health and the environment through the assessment of the risks from the deliberate release or the contained use of GMOs. In the unprecedented situation of public health emergency created by the COVID-19 pandemic, it is necessary that the protection of public health prevails. Therefore, it is necessary to grant a temporary derogation from the requirements concerning a prior environmental risk assessment and [“authorisation or” was omitted from the official English translation] consent under Directives 2001/18/EC and 2009/41/EC for the duration of the COVID-19 pandemic or as long as COVID-19 is a public health emergency. The derogation should be limited to clinical trials with investigational medicinal products [French version uses the term “médicaments expérimentaux” which translates as “experimental medicinal products”] containing or consisting of GMOs intended to treat or prevent COVID-19. During the period in which the temporary derogation applies, the environmental risk assessment and [“authorisation or” was omitted from the official English translation] consent under Directives 2001/18/EC and 2009/41/EC should not be a prerequisite for the conduct of those clinical trials.” Exhibit No. 7
This regulation was adopted under an emergency procedure, without prior examination in committee, and without any debate or amendment.
An MEP commented on this as follows:
"This new regulation provides that clinical trials on a vaccine or treatment aimed at combatting Covid- l9 containing or consisting of GMOs may begin without a risk analysis being carried out on transport, environmental release or injection of genetically modified organisms into humans. (...)
This dangerous text exempts manufacturers of these GMO-based treatments and vaccines from providing the environmental risk assessment and biosecurity study required prior to any request for clinical trials and to the marketing of such drugs that were until now required by the GMO legislation.” Exhibit No. 15
The consequence of the introduction of this Regulation is the removal of:
"all procedures for protection, risk analysis, control, follow-up, labelling and public information regarding the use, transport, environmental release, injection of genetically modified organisms and microorganisms into humans where research or clinical trials on a Covid-19 vaccine or drug are concerned. » Exhibit No. 18
Six associations have already filed an action for annulment forthwith of the said Regulation before the Court of Justice of the European Union, criticising it in the following terms:
"a dangerous experiment, both for participants in clinical trials and for the human population and the environment, and [they] require the immediate application of the precautionary principle, in accordance with the rule of law". Exhibit No. 18
On this topic, the former Research Director of Pfizer, Dr. Michael Yeadon, in collaboration with the renowned German doctor Wolfgang Wodarg, created a petition addressed to the European Medicines Agency (EMA):
“Together with the former Head of Research at Pfizer, Dr. Michael Yeadon, I submitted a request with the EMA, the European Medicines Agency responsible for EU-wide drug approval, on 1 December 2020 for the immediate suspension of all SARS CoV-2 vaccine studies, in particular the BioNtech/Pfizer study on BNT162b (EudraCT number 2020-002641-42).
We demand that the studies - for the protection of the life and health of the test subjects - not be continued until a study design is available that is suitable to address the significant concerns expressed by an increasing number of renowned scientists about the safety of the vaccine and the study design.
As petitioners, we demand on the one hand that, due to the well-known lack of accuracy of the PCR test, a serious study with so-called Sanger sequencing must be used. This is the only way to make reliable statements on the efficacy of a vaccine against Covid-19. Neither the risk of disease nor a possible vaccine benefit can be determined with the necessary certainty on the basis of the many different PCR tests of highly variable quality. For this reason alone, such tests of vaccines on humans are unethical per se.
Furthermore, we demand that it must be ruled out beforehand that risks already known from previous studies, some of which stem from the nature of coronaviruses, could have a dangerous effect. Our concerns focus in particular on the following points:
The formation of so-called "non-neutralizing antibodies" can lead to an exaggerated immune reaction, especially when test subjects are confronted with the real, "wild" virus after vaccination. This so-called antibody-dependent amplification, ADE, has long been known from experiments with corona vaccines in cats, for example. In the course of these studies, all cats that initially tolerated the vaccination well died after being infected with real coronaviruses. This overreaction is further encouraged by adjuvants.
The vaccinations are expected to induce antibodies against spike proteins of SARS-CoV-2. However, spike proteins also contain syncytin-homologous proteins, which are essential for the formation of the placenta in mammals such as humans. It is essential to exclude the possibility that a vaccine against SARS-CoV-2 triggers an immune reaction against syncytin-1, as otherwise infertility of indefinite duration in vaccinated women could result.
BioNTech/Pfizer's mRNA vaccines contain polyethylene glycol (PEG). 70% of people develop antibodies against this substance - this means that many people can develop allergic, potentially fatal reactions to the vaccination.
The study is much too short to allow a realistic estimation of the long-term effects. As in the narcolepsy cases after the swine flu vaccination, in the event of the planned emergency approval, long-term effects would only be observed when it was already too late for millions of vaccinated people. Governments plan to expose millions of healthy people to unacceptable risks and coerce them into vaccination through discriminatory restrictions on the unvaccinated.
Nevertheless, BioNTech/Pfizer clearly submitted an application for emergency approval on 1 December 2020. Scientific responsibility compels us to take this action.
CALL FOR AID: Dr. Wodarg and Dr. Yeadon ask as many EU citizens as possible to co-sign their petition by sending the e-mail prepared here to the EMA.” Exhibit No. 17
c) Knowledge of the risks and damages expected by the authorities: pharmaceutical laboratories and the medical profession and their already organized management
The scientific and medical community is well aware of the risks and damages to be expected as a result of this "vaccination" of the population.
§ Indeed, in a procurement contract award notice in the context of a contract awarded by the European Union, the following report is set out in the section entitled "description of the procurement":
“The MHRA [Medicines and Healthcare Products Regulatory Agency] urgently seeks an Artificial Intelligence (Al) software tool to process the expected high volume of Covid-19 vaccine Adverse Drug Reaction (ADRs) and ensure that no details from the ADRs’ reaction text are missed.” Exhibit No. 13
In other words, the UK Medicines and Healthcare Products Regulatory Agency (MHRA) is urgently seeking a company that can provide it with an artificial intelligence tool to deal with the particularly high and expected volume of adverse vaccine reactions, suggesting that their current data processing system will be insufficient to handle the number of requests.
Indeed, it is clearly stated a few lines later that:
“lt is not possible to retrofit the MHRA's legacy systems to handle the volume of ADRs that will be generated by a Covid-19 vaccine.”
These statements can be translated as follows:
“It is not possible to adapt the existing MHRA systems to handle the volume of adverse events that will be generated by a Covid-19 vaccine.”
Worse, the MHRA unequivocally states that the vaccine was launched before the completion of this artificial intelligence tool:
“The MHRA recognises that its planned procurement process for the SafetyConnect programme, including the Al tool, would not have concluded by vaccine launch. Leading to an inability to effectively monitor adverse reactions to a Covid-19 vaccine.”
Yet, this document states above:
“Therefore, if the MHRA does not implement the Al tool, it will be unable to process these ADRs effectively. This will hinder its ability to rapidly identify any potential safety issues with the Covid-19 vaccine and represents a direct threat to patient life and public health.”
The MHRA therefore makes it clear that vaccination against Covid-19 involves:
1. Serious adverse events that will affect a large number of people.
2. Such a large number of people affected by adverse events that artificial intelligence software will be required to deal with all the cases.
3. That the implementation of such software is necessary in order to ensure that no details of the side effects related to vaccination are forgotten.
4. That the implementation of such software cannot take place before the start of the vaccination plan.
5. That in the absence of such software, a direct threat exists to the lives of patients and public health.
The MHRA is therefore fully aware not only of the existence of adverse events from the Covid-19 "vaccine", but also of the fact that they are [likely to be] particularly numerous, and has been aware of this since at least 14 September 2020, the date of the conclusion of this contract!
However, it is this same entity, the MHRA, that approved on 2 December 2020, in full knowledge of the facts, the distribution of the gene therapy proposed by the Pfizer pharmaceutical group:
Of course, this phenomenon can only occur if genetic material from at least two viruses ends up in the same cells, which is fortunately extremely rare in nature since it implies that the same cells are co-infected with at least two viruses. But when viruses are man-made, this phenomenon can become much more common. This applies of course, as mentioned above, in the case of transgenic plants into which a viral transgene has been introduced when these plants need only be infected with a single virus for such recombination events to take place. But we run the same risk in humans when we generate vaccines introducing viral RNA or DNA into the cells of patients. Anti-Covid-19 vaccines of this type in clinical trials are administered intramuscularly or intradermally. The target cells are therefore muscle cells, skin cells and fibroblasts (cells of connective tissue, which means support tissue that envelops organs, tissues, and in particular muscular bundles) but also circulating blood cells and endothelial cells (which line the blood vessels), as well as many cells that can be the target of infections by other viruses. For example, enteroviruses (nonenveloped RNA viruses) have been detected in muscle cells [19], the Zika virus infects skin cells [20], the Chikungunya virus targets satellite muscle cells (muscle stem cells) [21], but also endothelial cells and fibroblasts [22]. And these are probably but a few examples ...
Vaccination against Covid-19, if it becomes a reality, will be a mass vaccination throughout the whole world. The probability of these kinds of events occurring is therefore far from being zero even if it is low in terms of frequency. Such mass vaccination with this type of vaccine could produce a huge number of new recombinant viruses. Let us not forget that it is enough for a single new virus to appear somewhere in the world for the health, environmental and social consequences to be global and vast ...
V.2. Genotoxicity: risk of insertional mutagenesis
Insertional mutagenesis is a mutation, in other words a modification of genetic information by inserting a sequence inside a genome, which can then inactivate or modify the expression of one or more genes.
This risk of genotoxicity for the human cells targeted by vaccination (whose genome is of course DNA) therefore concerns only vaccines delivering viral DNA, whether the vector is a plasmid or a genetically modified virus. However, this risk may also apply to vaccines delivering RNA through a genetically modified RNA viral vector such as the AIDS virus (HIV, widely used as a vector) if it has not been correctly stripped of its reverse transcriptase and the gene encoding it. Indeed, viral reverse transcriptase can then convert the delivered RNA into DNA, which will integrate into the genome of the target cells.
Genetically modified viruses are also widely used for gene therapy purposes to deliver in this case the normal version of a defective (mutated) human gene in the treated patient. In 2002, three years after a gene therapy trial (in children with severe immunodeficiency due to a mutation on an X chromosome gene) using a genetically modified RNA virus as a vector, two of the 10 children treated developed leukaemia due to the insertion of the repair DNA delivered by the viral vector near a proto-oncogene (cancer gene), causing a severe disruption in the expression of this [23]. Several studies have shown the effects of insertional mutagenesis caused by different families of RNA viruses {including HIV) [24]. Similarly, several studies in mice have shown that gene delivery by vectors derived from the adeno-associated virus (AAV, a small non-pathogenic DNA virus) results in insertional mutagenesis [25]. In 2016, a study on the genotoxic effects of viral vectors derived from HIV and AAV, used for gene therapy purposes, concluded that "a thorough understanding of viral biology and advances in cell genetics is needed in order to elucidate the nature of viral vector integration site selection and associated risks" [25 ].
V. lmmunotoxicity: risks specifically related to the use of modified viral vectors
In addition to the risks of occurrence of recombinant viruses and insertional mutagenesis, especially when the delivered genetic material is DNA, viral vectors can produce significant immunotoxicity effects given that they are themselves immunogenic. In 2002, a pilot gene therapy experiment carried out in 18 boys suffering from a severe metabolic disorder due to a failed gene located on the X chromosome led to the death of an 18-year-old man due to a fatal systemic inflammatory response caused by the viral vector (inactivated human DNA virus): DNA sequences of the vector were found in most of his tissues [27]. The fact that the other 17 persons treated did not show this type of response at all shows how difficult this risk is to predict and therefore difficult to overcome. In Belgium, several clinical trials of immunotherapy to fight cancer using an inactivated virus in which more than 15% of its genome was replaced by two human genes (encoding an antigen present on the surface of cancer cells and an interleukin, a protein that mediates communication between immune cells) showed a non-specific activation of the immune system linked to the vector resulting in an inflammatory reaction and an autoimmune response [28]. Many other studies have shown immunotoxicity effects of various viral vectors used for gene therapy or vaccination purposes [29-33]. In the case of viral vectors used for vaccination purposes, anti-vector immunity can also directly interfere with the desired vaccine efficacy (immunogenicity of the vaccine) [34].
V. General considerations for risk assessment of these vaccines
The use of vaccines that deliver viral genetic material (DNA or RNA) is new or recent. The use of genetically modified viruses as vectors, especially for gene therapy or immunotherapy, has shown the extent to which adverse reactions can be varied, uncontrolled and serious. Although immunotherapy efforts are relatively recent, we should not forget the failures of gene therapy over nearly 35 years.
These failures are largely explained by the race to the finish line at the expense of efficacy and/or biosecurity. Such an approach will never enable expectations or needs in terms of care to be met. (…)
Uncontrolled side effects would therefore have major repercussions, especially in a mass vaccination campaign such as the one to combat Covid-19. These repercussions could not only be disastrous in terms of health, of course, but also in terms of the environment, for example in the event of new recombinant viruses spreading. (See section IV.3.1.) And the fact that it is a preventive approach should not be a licence for risk-taking.
These vaccine candidates consequently require a thorough health and environmental assessment despite the urgency, whether arising from pressure from decision-making and health authorities or from the profit motive of the pharmaceutical industries embarked on this vaccine race. In its discussion paper of 23 July 2020 on the Covid-19 vaccine strategy [35], the National Authority for Health (HAS) stated: ‘In the context of the Covid-19 pandemic, the challenge is therefore to develop as effective and safe a vaccine as possible in record time.’ This exhortation on the part of an authority such as HAS is as nonsensical as it is outrageous.” Exhibit No. 1
This report makes edifying reading: the possible side effects and complications are very serious and may include death.
Yet despite the acknowledged dangers and side effects, the European Union has taken the liberty of removing the safeguards that it had itself imposed regarding the manipulation of genetically modified organisms (GMOs), as well as the requirements for environmental risk assessment and prior authorisation or consent specified by Directives 2009/41/EC and 2001/18/EC.
b) Establishment of a derogation procedure allowing the dissemination of vaccines without the issuance of a marketing authorisation and without evaluation by the scientific community
By Regulation 2020/1043 adopted by emergency procedure on 15 July 2020, in preambular paragraph 17, the European Union introduced a derogation regime for GMO manipulation and experimental medicinal products in these terms:
“(17) The main objective of Union legislation on medicinal products is to safeguard public health. That legislative framework is supplemented by the rules in Directive 2001/20/EC laying down specific standards for the protection of clinical trial subjects. Directives 2001/18/EC and 2009/41/EC have as their objective to ensure a high level of protection of human health and the environment through the assessment of the risks from the deliberate release or the contained use of GMOs. In the unprecedented situation of public health emergency created by the COVID-19 pandemic, it is necessary that the protection of public health prevails. Therefore, it is necessary to grant a temporary derogation from the requirements concerning a prior environmental risk assessment and [“authorisation or” was omitted from the official English translation] consent under Directives 2001/18/EC and 2009/41/EC for the duration of the COVID-19 pandemic or as long as COVID-19 is a public health emergency. The derogation should be limited to clinical trials with investigational medicinal products [French version uses the term “médicaments expérimentaux” which translates as “experimental medicinal products”] containing or consisting of GMOs intended to treat or prevent COVID-19. During the period in which the temporary derogation applies, the environmental risk assessment and [“authorisation or” was omitted from the official English translation] consent under Directives 2001/18/EC and 2009/41/EC should not be a prerequisite for the conduct of those clinical trials.” Exhibit No. 7
This regulation was adopted under an emergency procedure, without prior examination in committee, and without any debate or amendment.
An MEP commented on this as follows:
"This new regulation provides that clinical trials on a vaccine or treatment aimed at combatting Covid- l9 containing or consisting of GMOs may begin without a risk analysis being carried out on transport, environmental release or injection of genetically modified organisms into humans. (...)
This dangerous text exempts manufacturers of these GMO-based treatments and vaccines from providing the environmental risk assessment and biosecurity study required prior to any request for clinical trials and to the marketing of such drugs that were until now required by the GMO legislation.” Exhibit No. 15
The consequence of the introduction of this Regulation is the removal of:
"all procedures for protection, risk analysis, control, follow-up, labelling and public information regarding the use, transport, environmental release, injection of genetically modified organisms and microorganisms into humans where research or clinical trials on a Covid-19 vaccine or drug are concerned. » Exhibit No. 18
Six associations have already filed an action for annulment forthwith of the said Regulation before the Court of Justice of the European Union, criticising it in the following terms:
"a dangerous experiment, both for participants in clinical trials and for the human population and the environment, and [they] require the immediate application of the precautionary principle, in accordance with the rule of law". Exhibit No. 18
On this topic, the former Research Director of Pfizer, Dr. Michael Yeadon, in collaboration with the renowned German doctor Wolfgang Wodarg, created a petition addressed to the European Medicines Agency (EMA):
“Together with the former Head of Research at Pfizer, Dr. Michael Yeadon, I submitted a request with the EMA, the European Medicines Agency responsible for EU-wide drug approval, on 1 December 2020 for the immediate suspension of all SARS CoV-2 vaccine studies, in particular the BioNtech/Pfizer study on BNT162b (EudraCT number 2020-002641-42).
We demand that the studies - for the protection of the life and health of the test subjects - not be continued until a study design is available that is suitable to address the significant concerns expressed by an increasing number of renowned scientists about the safety of the vaccine and the study design.
As petitioners, we demand on the one hand that, due to the well-known lack of accuracy of the PCR test, a serious study with so-called Sanger sequencing must be used. This is the only way to make reliable statements on the efficacy of a vaccine against Covid-19. Neither the risk of disease nor a possible vaccine benefit can be determined with the necessary certainty on the basis of the many different PCR tests of highly variable quality. For this reason alone, such tests of vaccines on humans are unethical per se.
Furthermore, we demand that it must be ruled out beforehand that risks already known from previous studies, some of which stem from the nature of coronaviruses, could have a dangerous effect. Our concerns focus in particular on the following points:
The formation of so-called "non-neutralizing antibodies" can lead to an exaggerated immune reaction, especially when test subjects are confronted with the real, "wild" virus after vaccination. This so-called antibody-dependent amplification, ADE, has long been known from experiments with corona vaccines in cats, for example. In the course of these studies, all cats that initially tolerated the vaccination well died after being infected with real coronaviruses. This overreaction is further encouraged by adjuvants.
The vaccinations are expected to induce antibodies against spike proteins of SARS-CoV-2. However, spike proteins also contain syncytin-homologous proteins, which are essential for the formation of the placenta in mammals such as humans. It is essential to exclude the possibility that a vaccine against SARS-CoV-2 triggers an immune reaction against syncytin-1, as otherwise infertility of indefinite duration in vaccinated women could result.
BioNTech/Pfizer's mRNA vaccines contain polyethylene glycol (PEG). 70% of people develop antibodies against this substance - this means that many people can develop allergic, potentially fatal reactions to the vaccination.
The study is much too short to allow a realistic estimation of the long-term effects. As in the narcolepsy cases after the swine flu vaccination, in the event of the planned emergency approval, long-term effects would only be observed when it was already too late for millions of vaccinated people. Governments plan to expose millions of healthy people to unacceptable risks and coerce them into vaccination through discriminatory restrictions on the unvaccinated.
Nevertheless, BioNTech/Pfizer clearly submitted an application for emergency approval on 1 December 2020. Scientific responsibility compels us to take this action.
CALL FOR AID: Dr. Wodarg and Dr. Yeadon ask as many EU citizens as possible to co-sign their petition by sending the e-mail prepared here to the EMA.” Exhibit No. 17
c) Knowledge of the risks and damages expected by the authorities: pharmaceutical laboratories and the medical profession and their already organized management
The scientific and medical community is well aware of the risks and damages to be expected as a result of this "vaccination" of the population.
§ Indeed, in a procurement contract award notice in the context of a contract awarded by the European Union, the following report is set out in the section entitled "description of the procurement":
“The MHRA [Medicines and Healthcare Products Regulatory Agency] urgently seeks an Artificial Intelligence (Al) software tool to process the expected high volume of Covid-19 vaccine Adverse Drug Reaction (ADRs) and ensure that no details from the ADRs’ reaction text are missed.” Exhibit No. 13
In other words, the UK Medicines and Healthcare Products Regulatory Agency (MHRA) is urgently seeking a company that can provide it with an artificial intelligence tool to deal with the particularly high and expected volume of adverse vaccine reactions, suggesting that their current data processing system will be insufficient to handle the number of requests.
Indeed, it is clearly stated a few lines later that:
“lt is not possible to retrofit the MHRA's legacy systems to handle the volume of ADRs that will be generated by a Covid-19 vaccine.”
These statements can be translated as follows:
“It is not possible to adapt the existing MHRA systems to handle the volume of adverse events that will be generated by a Covid-19 vaccine.”
Worse, the MHRA unequivocally states that the vaccine was launched before the completion of this artificial intelligence tool:
“The MHRA recognises that its planned procurement process for the SafetyConnect programme, including the Al tool, would not have concluded by vaccine launch. Leading to an inability to effectively monitor adverse reactions to a Covid-19 vaccine.”
Yet, this document states above:
“Therefore, if the MHRA does not implement the Al tool, it will be unable to process these ADRs effectively. This will hinder its ability to rapidly identify any potential safety issues with the Covid-19 vaccine and represents a direct threat to patient life and public health.”
The MHRA therefore makes it clear that vaccination against Covid-19 involves:
1. Serious adverse events that will affect a large number of people.
2. Such a large number of people affected by adverse events that artificial intelligence software will be required to deal with all the cases.
3. That the implementation of such software is necessary in order to ensure that no details of the side effects related to vaccination are forgotten.
4. That the implementation of such software cannot take place before the start of the vaccination plan.
5. That in the absence of such software, a direct threat exists to the lives of patients and public health.
The MHRA is therefore fully aware not only of the existence of adverse events from the Covid-19 "vaccine", but also of the fact that they are [likely to be] particularly numerous, and has been aware of this since at least 14 September 2020, the date of the conclusion of this contract!
However, it is this same entity, the MHRA, that approved on 2 December 2020, in full knowledge of the facts, the distribution of the gene therapy proposed by the Pfizer pharmaceutical group:

§ Moreover, in an article dated 6 December 2020, the International Association for Independent and Caring Scientific Medicine (AIMSIB) made public an exchange between one of its members and the Ordre des médecins [French Medical Association]. Exhibit No. 14
In an email dated 30 November 2020, the Ordre des médecins [French Medical Association] responded to a member of the AIMSIB who raised the issue of vaccines in the following terms:
"Moreover, I think that politically a decision to make vaccination compulsory is very unlikely because this measure would be counterproductive and our governments and particularly the Minister of Health are aware of this.”
Consequently, the vaccination plan implemented in France and Europe is not only particularly dangerous for health and the environment, but also violates fundamental and constitutional rules of international law, which are the safeguards against such violations.
5. Violation of international and constitutional instruments
a) Violation of international instruments
The approval of the Pfizer/BioNTech vaccine by the European Union without prior analysis of the health and environmental risks violates numerous international instruments.
Indeed, article 5 of the Oviedo Convention [Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine[i]] provides that:
“An intervention in the health field may only be carried out after the person concerned has given free and informed consent to it.
This person shall beforehand be given appropriate information as to the purpose and nature of the intervention as well as on its consequences and risks.
The person concerned may freely withdraw consent at any time.” Exhibit No. 19
Moreover, article 6 [Consent] of the Universal Declaration on Bioethics and Human Rights of 19 October 2005 provides that:
“1. Any preventive, diagnostic and therapeutic medical intervention is only to be carried out with the prior, free and informed consent of the person concerned, based on adequate information. The consent should, where appropriate, be express and may be withdrawn by the person concerned at any time and for any reason without disadvantage or prejudice. Exhibit No. 20
However, no official information can clearly set out the risks or consequences of such a "vaccination" because no official studies have been conducted, such that the consent given can never be free or informed.
Paragraph 2 of article 3 of the same Declaration provides further that:
2. The interests and welfare of the individual should have priority over the sole interest of science or society. Exhibit No. 20
However, in view of the few studies conducted highlighting the potentially dramatic effects of these gene therapies, the interests and well-being of the individual are largely sacrificed on the alleged altar of science and the common good.
Worse, this "vaccine" seems to have been introduced especially in the interest of a few individuals: the bosses of pharmaceutical laboratories.
Indeed, between 15 May and 31 August 2020, the executives of five pharmaceutical companies earned more than 145 million [US] dollars from the sale of their shares. Exhibit No. 21
Article 16 of the same Declaration states:
“The impact of life sciences on future generations, including on their genetic constitution, should be given due regard.”
However, Professor Perronne clearly indicates that there is a risk of genetic transformation having an impact on the DNA of future generations:
"Thus an RNA foreign to our body and administered by injection could code for DNA, equally foreign, which can then be integrated into our chromosomes. There is therefore a real risk of transforming our genes definitively. There is also the possibility, by modifying the nucleic acids of our eggs or sperm, of transmitting these genetic modifications to our children.” Exhibit No. 22
The Nuremberg Code is a list of ten criteria contained in the judgment of the Nuremberg doctors' trial (December 1946-August 1947), indicating the conditions that must be met by experiments on human beings in order to be considered "acceptable":
“1. The voluntary consent of the human subject is absolutely essential. This means that the person involved should have legal capacity to give consent; should be so situated as to be able to exercise free power of choice, without the intervention of any element of force, fraud, deceit, duress, over-reaching, or other ulterior form of constraint or coercion; and should have sufficient knowledge and comprehension of the elements of the subject matter involved, as to enable him to make an understanding and enlightened decision. This latter element requires that, before the acceptance of an affirmative decision by the experimental subject, there should be made known to him the nature, duration, and
purpose of the experiment; the method and means by which it is to be conducted; all inconveniences and hazards reasonably to be expected; and the effects upon his health or person, which may possibly come from his participation in the experiment. The duty and responsibility for ascertaining the quality of the consent rests upon each individual who initiates, directs or engages in the experiment. It is a personal duty and responsibility which may not be delegated to another with impunity.
2. The experiment should be such as to yield fruitful results for the good of society, unprocurable by other methods or means of study, and not random and unnecessary in nature.
3. The experiment should be so designed and based on the results of animal experimentation and a knowledge of the natural history of the disease or other problem under study, that the anticipated results will justify the performance of the experiment.
4. The experiment should be so conducted as to avoid all unnecessary physical and mental suffering and injury.
5. No experiment should be conducted, where there is an a priori reason to believe that death or disabling injury will occur; except, perhaps, in those experiments where the experimental physicians also serve as subjects.
6. The degree of risk to be taken should never exceed that determined by the humanitarian importance of the problem to be solved by the experiment.
7. Proper preparations should be made and adequate facilities provided to protect the experimental subject against even remote possibilities of injury, disability, or death.
8. The experiment should be conducted only by scientifically qualified persons. The highest degree of skill and care should be required through all stages of the experiment of those who conduct or engage in the experiment.
9. During the course of the experiment, the human subject should be at liberty to bring the experiment to an end, if he has reached the physical or mental state, where continuation of the experiment seemed to him to be impossible.
10. During the course of the experiment, the scientist in charge must be prepared to terminate the experiment at any stage, if he has probable cause to believe, in the exercise of the good faith, superior skill and careful judgement required of him, that a continuation of the experiment is likely to result in injury, disability, or death to the experimental subject.”
The French government's vaccination plan violates all of these fundamental international texts, as well as the precautionary principle with constitutional value.
b) Violation of the precautionary principle
The precautionary principle is enshrined in article 5 of the [French] Charter on the Environment, which has been part of the constitutional framework since 2005, in these terms:
“When the occurrence of any damage, albeit unpredictable in the current state of scientific knowledge, may seriously and irreversibly harm the environment, public authorities shall, with due respect for the principle of precaution and the areas within their jurisdiction, ensure the implementation of procedures for risk assessment and the adoption of temporary measures commensurate with the risk involved in order to preclude the occurrence of such damage.”
While this principle has integrated the constitutional framework in an environmental context, it is also applicable in health matters.
Indeed, the precautionary principle has been affirmed in medicine, especially with the so-called "contaminated blood" case.
William Dab, Professor and Health and Safety Chair and responsible for teaching about occupational health and environmental risks at Cnam [Conservatoire National des Arts et Métiers, France], explains on this topic:
“The main public health lesson to be learned from the painful tainted blood affair is that in situations of uncertainty, decisions must be made, not on the basis of the more or less explicit advice of those claiming to be experts, but on the basis of a collective process of contradictory expertise, using explicit health criteria, letting people know in advance when it will be deemed that the problem is sufficiently understood to take action.” Exhibit No. 23
The precautionary principle is found more particularly in the medical field in article R4127-39 of the Public Health Code, which provides that:
"Doctors may not propose to patients or their relatives as beneficial or harmless a remedy or a procedure that is illusory or insufficiently tested. Any practice of charlatanism is forbidden.”
However, it has been explained that no contradictory expertise has been obtained. The procedure is therefore insufficiently tested and not without danger, in violation of the precautionary principle.
This is also the conclusion of the MEP Michèle RIVASI, who stated on 7 September 2020, during an interview with France Soir:
“The Commission specifies that this applies only to clinical trials, is valid only in the context of the fight against Covid-19, and as long as it is classified as a pandemic or considered a public health emergency. The fact remains that this proposal for a derogation from the GMO legislation for experimental GMO drugs against Covid-19 is a very bad signal for us Greens, contrary to the precautionary principle. Exhibit No. 15
The "vaccination plan" was implemented in violation of fundamental instruments representing, inter alia, safeguards for fundamental freedoms, and in particular the right to information, the right to safety and the right to life.
Through the implementation of this "vaccination plan", very many people are criminally liable on several grounds.
II – ACTS COMMITTED CAUSING DAMAGE TO THE PERSONS REPRESENTED BY THE REACTION 19 ASSOCIATION CONSTITUTE SERIOUS CRIMINAL OFFENCES
The provision and distribution of gene therapy products may be criminally classified as deliberate endangerment of the life of others (1), deception (2), extortion (3) or abuse of weakness (4).
1. The offence of deliberately endangering other persons
The offence of deliberately endangering other persons is provided for in article 223-1
of the Criminal Code:
“The direct exposure of another person to an immediate risk of death or injury likely to cause mutilation or permanent disability by the manifestly deliberate violation of a specific obligation of safety or prudence imposed by any statute or regulation is punished by one year's imprisonment and a fine of €15,000.”
In order to establish the crime of deliberate endangerment of other persons, it is necessary to identify an obligation of prudence or safety imposed by law or regulation (a), to demonstrate the deliberate violation of this obligation (b), as well as the existence of an immediate risk of death or serious injury (c).
a) The existence of a special duty of safety or prudence imposed by law or regulation
§ The right to information and the obligation to obtain free and informed consent before the performance of a medical procedure.
The Oviedo Convention on Human Rights and Biomedicine of 1997, in article 5, also requires that:
“An intervention in the health field may only be carried out after the person concerned has given free and informed consent to it.
This person shall beforehand be given appropriate information as to the purpose and nature of the intervention as well as on its consequences and risks. The person concerned may freely withdraw consent at any time.”
In addition, Article L1111-4 of the Public Health Code provides that:
"No medical procedure or treatment may be performed without the person's free and informed consent and such consent may be withdrawn at any time."
Article 16-3 of the Civil Code further provides that:
“There may be no infringement of the integrity of the human body except in case of medical necessity for the person or exceptionally in the therapeutic interest of another.
The consent of the interested person concerned must be obtained beforehand, except when his condition necessitates a therapeutic intervention to which he is not able to assent.”
Paragraph 1 of article R.4127-35 of the Public Health Code provides that:
“The doctor owes the person he examines, treats or advises fair, clear and appropriate information about his condition, the examinations and the care he offers. Throughout the disease, he takes into account the personality of the patient in his explanations and ensures that they are understood.”
Article R.4127-36 of the Public Health Code provides that:
"The consent of the person examined or treated must be sought in all cases.
When the patient, able to express his will, refuses the proposed investigations or treatment, the doctor must respect this refusal after informing the patient of its consequences.
If the patient is unable to express his will, the doctor may not intervene without the person of trust, failing which the family or one of his relatives having been warned and informed, unless in a case of emergency or impossible.
The obligations of the doctor towards the patient when the latter is a minor or a protected adult are defined in Article R. 4127-42. »
Indeed, any failure to comply with the duty to inform and the obligation to obtain free and informed consent deprives the patient of the opportunity to escape a risk.
§ The precautionary principle
In addition to this right to information and the obligation to obtain free and informed consent, there is a precautionary principle reflected in article R4127-39 of the Public Health Code.
Indeed, this article provides that:
“Doctors may not propose to patients or their relatives as beneficial or safe a remedy or procedure that is illusory or insufficiently proven. Any practice of charlatanism is forbidden.”
There are therefore several specific obligations imposed by law or regulation on medical personnel, concerning, on the one hand, the obligation to provide information and informed consent and, on the other hand, the precautionary principle.
§ The obligation of the State to guarantee everyone the right to health protection
There is also a legal obligation of the State to guarantee everyone the right to health protection.
Indeed, article L1411-1 of the Public Health Code provides that:
"The Nation defines its health policy in order to guarantee everyone the right to health protection.
Health policy is the responsibility of the State.
It aims to ensure the promotion of living conditions favourable to health, the improvement of the population's health status, the reduction of social and territorial inequalities and equality between women and men, and to guarantee the best possible health security and effective access of the population to prevention and care.
The health policy includes:
1. Monitoring and observation of the population's health status and the identification of its main determinants, particularly those related to education and living and working conditions. The identification of these determinants is based on the concept of the exposome, understood as the lifelong integration of all exposures that can influence human health;
2. Promotion of health in all living environments, particularly in educational institutions and in the workplace, and the reduction of health risks related to food, environmental factors and living conditions likely to degrade it;
3. Collective and individual prevention, throughout life, of diseases and pain, trauma and loss of autonomy, in particular through the definition of an educational path for children's health, through health education, through combatting sedentary lifestyles and through the development of the regular practice of physical and sports activities at all ages;
4. National coordination of actions carried out in the framework of the protection and promotion of maternal and child health referred to in article L. 2111-1;
5. Organization of health pathways. These pathways aim, through the coordination of health, social and medico-social actors, in connection with users and local authorities, to guarantee the continuity, accessibility, quality, safety and efficiency of population care, taking into account the geographical, demographic and seasonal specificities of each territory, in order to contribute to territorial equity;
6. The collective and solidarity-based coverage of the financial and social consequences of illness, accident and disability by the social protection system;
7. Preparedness and response to health alerts and crises;
8. Production, use and dissemination of knowledge useful for its development and implementation;
9. Promotion of training, research and innovation activities in the field of health;
10. Balancing initial and continuing training of health professionals and the exercise of their responsibilities;
11. Informing the population and ensuring its participation, directly or through associations, in public debates on health issues and health risks and in the processes of development and implementation of health policy.
Health policy is adapted to the needs of people with disabilities and their family caregivers.
Any draft law relating to health policy, with the exception of draft laws on the financing of social security and the finance law, is subject to prior consultation with the National Union of Health Insurance Funds, the professional bodies representing the mutual insurance companies and unions of mutual insurance companies governed by the Mutual Insurance Code, the provident institutions and unions of provident institutions governed by the Social Security Code, and the companies mentioned in Article L. 310-1 of the Insurance Code and offering guarantees relating to the reimbursement and compensation of expenses incurred due to illness, maternity or accident, the National Union of Health Professionals, representatives of local authorities and the National Union of Approved Health System Users' Associations.”
b) Deliberate breach of specific duties of care imposed by the law or regulation
The deliberate violation of this obligation constitutes the intentional element of the offence of endangering the life of other persons.
While it has been established that the human health effects of messenger RNA technology could be dramatic, the announcements made in recent weeks by President Macron and the Ministry of Health reflect the existence of an ongoing "vaccine strategy".
Indeed, President Macron, during his speech on 24 November 2020, indicated that a vaccination campaign would begin "from the end of December, beginning of January" for "the most fragile people".
The Minister of Health, meanwhile, announced that the French government had purchased essential equipment for the storage of "vaccines".[ii]
The government, represented by Prime Minister Jean Castex at the Ministry of Health press briefing on 3 November 2020, presented a vaccination plan that had already been precisely established in three stages.
“[It is recommended] to first vaccinate elderly people in institutions, particularly EHPADs [residental care homes for the elderly]. That represents about 1 million people.”
"Then, as the deliveries progress, we will expand the scope of vaccination, starting with the 14 million with age-related or chronic disease risk factors [...]. This is Stage 2 of our plan, which will begin in February and run until spring.”
"Finally, we will gradually open vaccination to the entire population from spring onwards. This will be Stage 3 of our strategy.” [iii]
Finally, a document entitled "Strategy for vaccination against Sars-Cov-2" was published by the National Authority for Health on 27 November 2020.
Thus, a veritable "vaccine strategy" was developed with a precise timetable, a first target audience defined and logistical arrangements were implemented.
Yet the Government implemented this action plan knowing the potentially devastating effects of a gene therapy and taking care not to communicate them to the general public.
Indeed, on the one hand it could not be unaware of the particularly revealing public study published by CRllGEN (Exhibit No. 6), or the open letter from Professor Perronne (Exhibit No. 22), Head of the Infectious and Tropical Diseases Department at Garches hospital, in which he stated on 30 November 2020:
“The people who promote these gene therapies, falsely called “vaccines,” are sorcerer’s apprentices and are treating the French and more generally the citizens of the world as guinea pigs.”
Thus, the Government and relevant medical stakeholders are deliberately depriving patients of their right to information, which will subsequently prevent them from providing informed consent.
Moreover, the Scientific Council stated in an opinion of 9 July 2020 that it was not recommending compulsory vaccination, but nor was it planning "a vaccination strategy based on purely individual choices". Exhibit No. 26
In addition, they are misleading the public by referring to a "vaccine" when in fact it is a gene therapy and will endanger a healthy population by injecting them with a potentially life-threatening product.
This erroneous use [of terminology] alone demonstrates the perpetrators' desire not to fulfil their particular obligation of information, prudence and safety, and actually to deliberately violate it by providing only partial information.
This particular violation was, moreover, highlighted by the International Association for Independent and Caring Scientific Medicine (AIMSIB) in an exchange made public between one of its members and the Ordre des médecins [French Medical Association] on 30 November 2020, in the following terms:
"Dear President and colleague,
Thank you for your second answer, which unfortunately is unsatisfactory from both the collegial and ethical points of view, as well as from a legal standpoint, and even more so from a scientific one.
1. You accuse me of “anti-vaccine rhetoric" just because I express serious reservations about these new products. This disdainful terminology undoubtedly indicates your very poor opinion of me. Others before you resorted to similar expressions such as "negro music", "communist film", "Jewish literature" or "degenerate art". Those did not end well, so "anti-vaccine rhetoric" should be greeted in the current climate as a sure sign of your knee-jerk rejection of the subject without your feeling the need to give it any thought.
2. You talk to me about "the rule of law, free and responsible choice to refuse care": I think you have forgotten the 2018 episode when infant immunization was made mandatory for eleven vaccines against the advice of the College of Health Professionals, I don't feel that parents have been able to choose freely since then, in the sense that you intend it. As for the free will of the institutionalized residents in EHPAD [residental care homes for the elderly] to receive an anti-Covid vaccination after clear and appropriate information … Is this black humour or are you really convinced of what you are saying? The government could not care less about shortening the lives of this captive population and is once again banning any collection of data on serious long-term adverse effects. Who has seriously studied the effects of influenza co-vaccination in the elderly? Is this a new hidden Stage III, theoretically absolutely prohibited? (2)(3)
3. “No really effective therapy against Covid": Your position is partisan, pro-industry, perfectly aligned with the government but light years away from the scientific reality described everywhere in the world. On the contrary, there is a plethora of products effective against Covid, both preventive and curative, all the data have been published: Vitamin D3, HCQ, azithromycin, zinc, artemisinin, ivermectin and right now, even the combination of quercetin-Vit.C-bromelain seems to be demonstrating a result at least equal to the Pfizer vaccine, here is an original pre-print from The Lancet on a Turkish study:
https://papers.ssrn.com/sol3/papers.cfm ?abstract_id=3682517
You can also read here:
https://blogs.mediapart.fr/laurent-mucchielli/blog/021220/l-importance-du-traitement-precoce-des-patients-ages-atteints-de-la-covid-en-ehpad always up to date.
4. "The documented results show real vaccine efficacy": Forgive me for saying so, but your statement is absolutely appallingly anti-science, humiliating for your institution, read the last two AlMSIB articles again:
https://www.aimsib.org/2020/11/22/vaccins-anti-covid-en-2020-folie-sanitaire-politique-mediatique-financiere/
https://www.aimsib.org/2020/l1/29/vaccins-anti-covid-surs-et-efficaces-avis-du-conseil-scientifique-de-la-has-ce-quen-a-fait-la-commission-europeenne/
Absolutely zilch that is scientifically admissible has been published anywhere about mRNA products, two of which are veritable snake-oil concoctions fit for fairgrounds. You are confusing genuine science with an advertising leaflet; a court of law will never understand how the Medical Association could have endorsed such a boondoggle. Have you forgotten that Pfizer was fined 2.3 billion dollars in 2009 for false advertising and you take this firm’s hype at face value? It's monumentally disappointing but unfortunately totally predictable and I’d been expecting it from my first email, because someone must be making you talk this way.
5. “Even if the vaccine is recent and the appraisal time short": You can be sure that no criminal lawyer will ever be content with a sentence like this to absolve vaccinators of their massive liability as soon as the first claims of misinformation and violation of art. 39 (which you carefully avoid citing) appear. Right now, these vaccines are not recent because they do not yet exist, they do not even have a marketing authorisation in Europe, and CNOM [Conseil national de l'Ordre des médecins - National Medical Council of the French Medical Association] is already endorsing them, but on whose orders? Before long this will be unravelling in court, you will then have to defend such a position in front of lawyers.
I am not very optimistic going forward, the health scandal will explode very quickly because the judges have already begun their work of enquiries and searches at the highest level of the State. Many judges and criminal lawyers believe the Covid-masks-HCQ-remdesivir-vaccine case will be the scandal of the century, a thousand times worse than the contaminated blood scandal. I don’t envy your position between the hammer and the anvil, perhaps an orchestrated resignation of all the departmental councils could help to ensure that the independence of medicine is finally recognised and saved, you would at least avoid the awkward consequences for you and your teams.
I am attaching only a very meagre list of articles for you to skim because I know from experience that the members of the ordinal councils (departmental, disciplinary, national, etc.) do not generally read anything that is sent to them. I will circulate your answer while anonymising your name and title, it is not a question of embarrassing you personally to our readers, our criticisms are actually aimed at your institution.
I nevertheless sign off in a collegial spirit. Yours, etc.” Exhibit No. 14
Violation by the Government and the medical profession of the duty to provide information, of the precautionary principle and of the State's obligation to guarantee the right to health protection for everyone is thus established.
a) The existence of an immediate risk of death or serious injury to other persons
Article 223-1 of the Criminal Code implies demonstrating that other persons are exposed to a "direct and immediate risk". It is therefore not necessary to demonstrate the existence of actual harm, but only that the reckless conduct is "of such a nature as to" cause harm.
As has been set out above, the injection of a gene therapy product into the human body is likely to have particularly severe effects on humans, including paralysis, cancer or death.
The offence of deliberate endangerment of another person is thus established in all its elements.
2. The offence of deception
The offence of deception is provided for in article L213-1 of the Consumer Code in the following terms:
“Anyone who, whether or not a party to the contract, has deceived or attempted to deceive the contractor by any means whatsoever, even through a third party, shall be punished by imprisonment for a maximum of two years and a fine of 300,000 euros:
1. Either on the nature, the type, the origin, the essential qualities, the composition or the content of necessary ingredients of all goods;
2. Either on the quantity of the goods delivered or on their identity by the delivery of goods other than the specific goods that were the subject of the contract;
3. Either on the suitability for use, the risks inherent in the use of the product, the controls carried out, the instructions for use or the precautions to be taken.
4. The amount of the fine may be increased, in proportion to the benefits derived from the breach, to 10% of the average annual turnover, calculated on the last three annual turnover figures known at the date of the acts.”
a) The substance of the offence of deception
The substance of the offence of deception presupposes, on the one hand, the use of means likely to deceive and, on the other hand, the carrying out of the deception.
Deception may relate to the essential qualities of any goods, as well as to the risks inherent in their use and the precautions to be taken in this respect.
In the present case, it has already been explained that products presented as vaccines are in fact gene therapies.
Yet the Government has knowingly used the misleading term "vaccines" instead of the scientifically correct term "gene therapy", and has established a communication campaign to that effect.
Moreover, the deception is effected because most French people today are unaware that the injection that they have planned, or not, to receive is actually a gene therapy.
The Government and the pharmaceutical companies are therefore deceiving the population by passing off a medical product as something that it is not.
c) The element of intent of the offence of deception
The element of intent of the offence of deception is established when the individual was aware that he or she was describing the incriminated product inaccurately.
In this case, the manufacturers of gene therapy products, as health professionals, cannot be unaware of the fact that these products are not vaccines and of the dangers they pose to health.
Furthermore, it follows from the explanations given in the introduction, and in particular from points 3 and 4, that the Government is aware that this is not a vaccine but a gene therapy, and of the potentially dramatic effects of the latter.
In this respect, by using the term "vaccine", the government and the pharmaceutical companies know that they are misleading the public.
Thus, the geneticist and former Director of Research at Inserm [French National Institute for Health and Medical Research], Alexandra Henrion-Caude stated in an interview published on 11 December 2020 on the Sputnik France website:
"Moreover, even under the pretext of a health emergency, which so many free people, without any conflict of interest, no longer believe in, how dare we play on people's credulity by using technocratic definitions of words? So ask people what a "vaccine" means to them. They will certainly not imagine that, through this injection, their body will become such a GMO, inheriting the genetic information of a virus that will force their cells to produce its viral protein in order to create antibodies directed against the cells that have produced the virus' protein, in a type of autoimmune reaction.
So we must stop using the word ‘vaccine’, which has been misappropriated by the regulatory legislation, and put in place a truly informed consent.” Exhibit No. 16
The substance of intent having been demonstrated, the offence of deception is established in all its elements.
3. The offence of fraudulent abuse of a person’s ignorance or weakness
Fraudulent abuse of a person’s ignorance or weakness is provided for in article 223-15-2 of the Criminal Code:
“Fraudulently abusing the ignorance or state of weakness of a minor, or of a person whose particular vulnerability, due to age, sickness, infirmity, to a physical or psychological disability or to pregnancy, is apparent or known to the offender, or abusing a person in a state of physical or psychological dependency resulting from serious or repeated pressure or from techniques used to affect his judgement, in order to induce the minor or other person to act or abstain from acting in any way seriously harmful to him, is punished by three years' imprisonment and a fine of €375, 000.”
a) Preconditions for the offence of fraudulent abuse of a person’s ignorance or weakness
Article 223-15-2 of the Criminal Code refers to three categories of protected persons: the minor, the person in a situation of particular vulnerability and the person in a state of psychological subjection.
The situation of particular vulnerability can, according to this law, be associated in particular to the age of the person, to an illness, infirmity or physical or psychological disability.
In this case, the strategy developed by the National Authority for Health (HAS) and made public on 30 November 2020 provides for:
“In this initial phase, when a very limited number of doses will be available, [some] populations appear to be a top priority because of their vulnerability (age and/or co-morbidities) and their increased exposure to the Sars-Cov-2 virus:
- Residents of institutions for the elderly and residents in long-stay services (EHPAD [residental care homes for the elderly] …)." Exhibit No. 24
In addition, the National Union of Private Retirement Homes and Residences for Seniors said in reference to EHPAD [residental care homes for the elderly] residents:
"40% to 60% of nursing home residents can no longer make decisions about their health because of serious illnesses such as Alzheimer's disease or dementia.” Exhibit No. 25
Consequently, the vaccination policy is primarily aimed at residents of EHPAD [residental care homes for the elderly] who are particularly vulnerable due to their age, illness, infirmity, and physical or mental impairment.
b) The substantive element of the offence of fraudulent abuse of the state of ignorance or weakness
In order to establish this offence, the fraudulent abuse of the state of ignorance or the situation of weakness must be shown to have led the person to an act or omission that is seriously prejudicial to him or her.
The perpetrator must have taken advantage of the person's ignorance or weakness to lead him or her to an act or omission that is seriously prejudicial to him or her. The act to which the vulnerable person has been led may be both substantive and legal.[iv]
c) Criminal law does not require the damage to have actually occurred.[v]
In this case, the residents of EHPADs [residental care homes for the elderly], who are particularly vulnerable, are in a situation of weakness that can be abused for the purpose of their consenting to the injection of gene therapy products which, as shown above, will cause side effects with particularly serious implications for their health.
c) The mental element of the offence of abuse of weakness
In order for the mental element of the crime of abuse of weakness to be established, the perpetrator must have been aware of the victim's state of ignorance or weakness and must have wanted to exploit it to obtain from the victim an act or omission which he or she knew to be of a seriously prejudicial nature.
In this case, "Stage 1" of the "vaccination" plan will begin in EHPADs [residental care homes for the elderly].
Consequently, the state of dependence and weakness of the persons receiving the doses of the product is known.
Furthermore, as explained above, several studies have shown that gene therapy products falsely referred to as "vaccines" will cause many serious side effects that are known to health care professionals.
The offence of abuse of weakness is thus established in all its elements.
4. The offence of extortion
The offence of extortion is provided for in article 312-1 of the Criminal Code, which states:
“Extortion is the act of obtaining by violence, by the threat of violence or constraint either a signature, a commitment or a renunciation, or the revelation of a secret, or the handing over of funds, securities or of any asset.
Extortion is punished by seven years' imprisonment and a fine of €100,000.”
Furthermore, article 312-2 of the Criminal Code specifies:
“Extortion is punished by ten years' imprisonment and a fine of €150,000:
1. when it is preceded, accompanied or followed by acts of violence upon other persons and which have caused a total incapacity to work for eight days or less;
2. when it is committed to the prejudice of a person whose particular vulnerability, due to age, sickness, infirmity, a physical or psychological disability or to pregnancy, is apparent or known to the perpetrator (…) ;
a) On the substantive element of extortion
In this case, it appears that the authorities are subjecting the population to mental coercion so that they agree to being vaccinated.
On the one hand, the government is creating a climate of fear and guilt in order to mentally coerce the population to be vaccinated.
To that end, the Ministry of Health produced particularly guilt-inducing advertisements.[vi] Exhibit No. 29
Moreover, the President of the Republic has been using the terminology of war in all his speeches since the beginning of the epidemic.
He has conveyed the idea of our being at war in his various speeches by asserting that "we are at war", by imposing a "curfew", by asserting that "the enemy is here, invisible and elusive", that health care workers are "in the front line of this fight", and so on.
In addition to this mental coercion based on fear and guilt, another form of constraint is being put in place, which consists of preventing those not vaccinated against Covid-19 from entering certain public places.
Indeed, more and more organizations are talking about a "vaccination passport", without which it will be impossible to be in certain public places or to travel.
That is what Christophe BARBIER, the former Editor of L’Express, confirmed when he said:
“If you are not vaccinated, you won't be able to go to restaurants or theatres, or take a plane ... you will need a vaccination certificate like a passport for entry into society.” Exhibit No. 30
His statement has already been translated into a practical application in the aviation field.
For the International Air Transport Association (IATA), which represents 290 airlines comprising 82% of global air traffic, issued a press release on 23 November 2020 in which it announced:
"The airline industry demands a cost effective, global, and modular solution to safely restart travel. IATA Travel Pass is based on industry standards and IATA’s proven experience in managing information flows around complex travel requirements.
· IATA’s Timatic is used by most airlines to manage compliance with passport and visa regulations and will be the base for the global registry and verification of health requirements.
· IATA’s One ID initiative was endorsed by a resolution at its 75th Annual General Meeting in 2019 to securely facilitate travel processes with a single identity token. It is the base for the IATA Contactless Travel App for identity verification that will also manage the test and vaccination certificates.
“Our main priority is to get people traveling again safely. In the immediate term that means giving governments confidence that systematic COVID-19 testing can work as a replacement for quarantine requirements. And that will eventually develop into a vaccine program. The IATA Travel Pass is a solution for both. And we have built it using a modular approach based on open source standards to facilitate interoperability. It can be used in combination with other providers or as a standalone end-to-end solution. The most important thing is that it is responsive to industry’s needs while enabling a competitive market … The first cross-border IATA Travel Pass pilot is scheduled for later this year and the launch slated for quarter one 2021.” Exhibit No. 31
The Government is thus subjecting the population to mental coercion coupled with a physical constraint consisting of making it impossible for them to be in certain facilities or to travel.
In addition, the International Association for Independent and Caring Scientific Medicine (AIMSIB) stated in its article published on its website on 29 November 2020:
“Vaccination will not be compulsory, but we can trust the French health authorities not to really leave a free individual choice to the citizens. This is all the more serious as the new technologies of future vaccines (never before used) add a lot of uncertainty about the safety and efficacy problems of future vaccines.”
AIMSIB states in this respect that:
"The European Commission ... has just finished signing six secret and outrageous contracts with vaccine manufacturers for a number of doses corresponding to the complete vaccination of 1.2 billion people". Exhibit No. 26
In addition, if the "vaccination" plan primarily concerns the elderly and those placed in EHPAD [residental care homes for the elderly], judges must take into account the victim's person, age, physical and intellectual condition and vulnerability in order to identify the substance of the offence.
Some among the elderly will have experienced a real state of war, such that the terminology empoyed by the President of the Republic makes them relive the terror they experienced.
Moreover, it is also the elderly who spend the most time watching television.
Indeed, according to a survey conducted by Nielsen in the United States, the over-fifties spend an average of 7 hours a day in front of the television. Exhibit No. 32
The elderly are thus the primary target audience for the commercials produced by the Ministry of Health that include, in particular, an elderly person in intensive care after kissing her grandchildren. Exhibit No. 29
In the light of the number of vaccines ordered and the view expressed by AlMSIB, the vaccination plan applies not only to the elderly and to those at risk, but to the entire population, the majority of whom are reluctant to be "vaccinated".
Indeed, the European Commission published a roadmap for vaccination in the third quarter of 2019, which clearly sets out the reluctance of the population to be vaccinated. Exhibit No. 38
Even more significantly, the same document calls for the introduction of a common vaccination card by 2022.
This last item clearly demonstrates the existence of a common vaccination strategy, enforceable on everyone.
By subjecting the population to mental coercion therefore, the Government intends to obtain their commitment to undergoing gene therapy.
b) The element of intent of the offence of extortion
The element of intent of the offence of extortion is established "by consciously obtaining by force, violence or coercion what could not have been obtained by an agreement freely entered into.”[vii]
It was established by the European Commission, in the roadmap on vaccination from the third quarter of 2019, that the European population is resistant to traditional vaccination. Exhibit No. 38
Yet a "gene therapy" is now under discussion, which has been shown to be novel, to have not been subject to proper consideration and to thereby involve risks.
There is therefore no doubt that the European population is all the more resistant to this new technology and recent polls underline this. Exhibit No. 12
Being aware of this resistance, the Government is employing a strategy designed to terrorise the population, and will soon impose a ban on travel and access to certain public places in order to coerce the population to adopt this gene therapy.
Indeed, in the light of all the studies conducted and the risks identified, as well as the strategy that it put in place, there is no doubt that the Government was aware that it could not obtain the population's agreement without exercising this coercion.
The offence of extortion is therefore established.
Furthermore, extortion, "when it is committed to the prejudice of a person whose particular vulnerability, due to age, illness, infirmity, a physical or psychological deficiency or a state of pregnancy, is apparent or known to the perpetrator", is aggravated.
The offence of extortion is thus established.
In this case, this crime is committed first and foremost against the elderly placed in EHPADs [residental care homes for the elderly] and presenting comorbidities, that is, suffering from a disease pre-existing the infection.
Their status of advanced age and illness cannot be unknown to the Government since these conditions represent the very grounds for their intervention.
The offence of aggravated extortion is thus established in all its elements.
The Public Prosecutor's Office is requested to open an investigation into the facts set out above constituting the following offences:
- The offence of deliberately endangering other persons
Article 223-1 of the Criminal Code
- The offence of aggravated deception
Articles L213-1 and L213-2 of the Consumer Code
- The offence of abuse of a person’s weakness
Article 223-15-2 of the Criminal Code
- The offence of aggravated extortion
Article 312-2 of the Criminal Code
The attention of the Public Prosecutor's Office is drawn to the urgency of conducting a criminal investigation, which is the only way to bring about the cessation of the crimes perpetrated against the victims.
The Réaction 19 Association stands ready to assist the investigating authorities in order to be heard on these facts and to provide any clarification that could be useful in establishing the truth.
Done in [Paris]
Law 1901 Association
On [16 December 2020]
No. P.W751256495
[Signature[viii]]
LIST OF EXHIBITS
1. Article published on the website of Médiapart, 8 December 2020: “Malgré les gesticulations du gouvernement, les Français rejettent le vaccin à ARN” (Despite government gesticulations, the French reject the RNA vaccine).
2. Article published on the website of Gala, 4 December 2020. “VIDEO – « C'est un gag ! » : Pascal Praud ironise sur le vaccin contre le Covid.” (VIDEO - "It's a gag!": Pascal Praud ironizes on the Covid vaccine).
3. Article published on the website of Tvlibertés, 9 December 2020. “Covid-19: 4 volontaires auraient développé une paralysie faciale temporaire après avoir reçu le vaccin Pfizer contre le coronavirus” (Covid-19: 4 volunteers developed facial paralysis after having received the Pfizer vaccine against coronavirus).
4. Article published on the website of Marseillenews.net, 9 December 2020. “Des experts américains se réunissent pour l’approbation du vaccin de Moderna” (American experts meet to approve the Moderna vaccine), 17 December 2020.
5. Article published on the website of France 24, 3 September 2009. “Le laboratoire Pfizer écope d'une amende de 2,3 milliards de dollars” (Pfizer laboratory receives a fine of 2.3 billion dollars).
6. CRIIGEN: Expert’s Note, September 2020 [Note d’expertise grand public sur les vaccins ayant recours aux technologies OGM – General Public Expert Note on Vaccines Using GMO Technologies].
7. Regulation (EU) 2020/1043 of the European Parliament and of the Council of 15 July 2020 on the conduct of clinical trials with and supply of medicinal products for human use containing or consisting of genetically modified organisms intended to treat or prevent coronavirus disease (COVID-19).
8. Article published on the website of Capital, 9 November 2020. [Not found]
9. Report published by lmperial College, London of 29 October 2020: Report 34 - COVID-19 Infection Fatality Ratio Estimates from Seroprevalence.
10. Video interview of Prof. Didier Raoult, 2 June 2020 (9 mins. 30). Les Pieds nickelés font de la science (Lazy people are doing science).
11. Etude publiée par US National Library of Medicine, National Institute of Health on 15 July 2020. [Not found]
12. Survey published on the website of BFMTV, 9 December 2020 (Sondage BFMTV - Près d’un Français sur deux ne compte pas se faire vacciner, une proportion en baisse – BFMTV survey nearly one in two French people are not planning to be vaccinated, a declining proportion, 17 December 2020).
13. Contract award notice published on the official website of the European Union on 19 October 2020.
14. Article published by AIMSIB, 6 December 2020.
15. Article published on the website of France Soir, 7 September 2020. “Les vaccins OGM, retour sur une décision en catimini au Parlement européen.” (GMO vaccines, the European Parliament's sneaky decision reversed).
16. Article published on the website of Sputnik News, 11 December 2020: “Vaccins contre le Covid-19: «une cascade très négative» d’effets secondaires à craindre?” (Vaccines against Covid-19: "a very negative cascade" of side effects to be feared?).
17. Article published on the website of France Soir, 3 December 2020. “Vaccination SARS-CoV-2 : le Dr Wodarg et le Dr Yeadon disent stop !” (SARS-CoV-2 vaccination: Drs. Wodarg and Yeadon say stop!).
18. Article published on the website of France Soir, 19 October 2020. “Vaccin : recours en annulation contre le règlement européen 2020/1043” (Vaccine: Action for annulment against European regulation 2020/1043).
19. Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine, Oviedo, 4.4.1997.
20. Universal Declaration on Bioethics and Human Rights of 19 October 2005.
21. Article published on the website of Capital, 15 November 2020. “Course au vaccin : l'enrichissement des patrons de laboratoires en question.” (Vaccine Race: Enrichment of Pharma CEOs Criticised).
22. Article published on the website of Putsch, 2 December 2020. [Found on the Le Monde website, 15 December 2020] “Les vaccins à ARN messager ne modifient pas l’ADN de nos cellules” (Messenger RNA vaccines do not change the DNA of our cells)
23. Note sur le principe de précaution publié par le Natures Sciences Sociétés en 1995.
24. Recommendation of the Haute Autorité de Santé [National Authority for Health] of 27 November 2020. “Stratégie de vaccination contre le Sars-Cov-2 - Recommandations préliminaires sur la stratégie de priorisation des populations à vacciner.” (Immunization strategy against Sars-Cov-2 - Preliminary recommendations on the prioritization strategy for populations to be vaccinated.)
25. Article published on the website of Medisite, 10 December 2020. “Covid : la vaccination inquiète dans les EHPAD.” (Covid: vaccination a cause for concern in EHPADs [residental care homes for the elderly].)
26. Article published on the website of AlMSIB, 29 November 2020. “Vaccins anti-Covid, sûrs et efficaces ? Avis du Conseil Scientifique, de la HAS, ce qu’en a fait la Commission Européenne.” (Are anti-Covid vaccines safe and effective? Opinion of the Scientific Council and HAS, and what the European Commission have done with them.)
27. Avis du Comité scientifique of 9 July 2020. Vaccins contre le SARS-CoV-2, 9 juillet 2020, Une stratégie de vaccination, CARE – Comité scientifique COVID-19 – Comité Vaccin COVID-19 (Covid-19 Scientific Committee: Vaccines against SARS-CoV-2, A strategy of vaccination).
28. Article published on the website of Eurodif, 2 November 2020. [Not found]
29. Television advertisement by the Ministry of Health published on Youtube on 12 September 2020: “Ministère des Solidarités et de la Santé ‘continuons d'appliquer les gestes barrières’ Pub 62s" (“Ministry for Solidarity and Health ‘Let’s continue to apply preventive measures’”).
30. Article published on the website of 20 Minutes, 17 November 2020. [Not found. Quotation confirmed at “Coronavirus : un certificat de vaccination bientôt mis en place ?” (Coronavirus: a vaccination certificate soon to be implemented?)]
31. Official IATA press release of 23 November 2020: IATA Travel Pass Key to Reopening Borders Safely.
32. Article published on the website of Yahoo style, 30 August 2019. [Not found. Found on Nielsen website, 31 July 2018: “Time Flies: U.S. Adults Now Spend Nearly Half a Day Interacting with Media.”]
33. Article published on the website of Sud Radio, 16 November 2020. Alexandra Henrion-Caude : "J'ai l'impression qu'on est revenu au temps des devins" (Alexandra Henrion-Caude: We seem to have regressed to the time of soothsayers).
34. Article published on the website of MesVaccins.net, 22 November 2020. “L'ARN est-il l'avenir des vaccins ?” (Is RNA the future of vaccines?)
35. Article published on the website of l’AIMSIB, 22 November 2020. [No Exhibit 35 appears in this document. See, however, in-text note 35 and Exhibit 26, which both refer to HAS.]
36. Décision Salvetti c/ Italie de la CEDH of 9 July 2002. [No Exhibit 36 appears in this document.]
37. Note publiée par l’Agence Européenne des médicaments en 2016. [No Exhibit 37 appears in this document.]
38. European Commission: Roadmap On Vaccination (Last update: Q3 2019).
[i] [“This Convention is the only international legally binding instrument on the protection of human rights in the biomedical field. It draws on the principles established by the European Convention on Human Rights, in the field of biology and medicine.
It is a framework Convention aiming at protecting the dignity and identity of all human beings and guarantee everyone, without discrimination, respect for their integrity and other rights and fundamental freedoms with regard to the application of biology and medicine.
It sets out fundamental principles applicable to daily medical practice and is regarded as such at the European treaty on patient’s rights. It also deals specifically with biomedical research, genetics and transplantation of organ and tissues.
The provisions of the Convention are further elaborated and complemented by Additional Protocols on specific subjects.”]
[ii] Minister of Health press briefing of 12 November 2020.
[iii] Video press conference of 3 November 2020 https://www.francetvinfo.fr/sante/maiadie/coronavirus/vaccin/video-Covid-l9-decouvrez-les-trois-phases-du- plan-de-vaccination-devoile-par-le-gouvernement_4205753.html.
[iv] Cass. Crim., 19 fév. 2014. n° 12-87558.
[v] Cass. Crim., 12 janv. 2000.
[vi] Ministère des Solidarités et de la Santé "continuons d'appliquer les gestes barrières" Pub 62s" https://www.youtube.com/watch?v=kHSsIoSZSQI. (“Ministry for Solidarity and Health - ‘Let’s continue to apply preventive measures’”.)
[vii] Crim., 9 janvier 1991, Bull. Crim. n° 17.
[viii] [Me Alberto Brusa interview on the subject of the present complaint (in French): https://www.youtube.com/watch?v=DKlTtWHjneg.]
