Should UN staff and other international civil servants take a Covid vaccine?
Letter from UN pensioner to UN insurer Allianz regarding cover for medical care for side-effects from Covid vaccines, 11 April 2021
UPDATES FEBRUARY 2022
Life insurance company bails out after vaccine death because "voluntary vaccination with experimental vaccine“ counts like suicide! 13.1.22
The die-off is here: life insurance payouts skyrocket 258% as post-vaxxine deaths rapidly accelerate, 1.2.22
Deaths up 40% among those aged 18-64 based on life insurance claims for 2021 after Covid-19 vaxxine roll outs, 3.1.22
Life insurance company bails out after vaccine death because "voluntary vaccination with experimental vaccine“ counts like suicide! 13.1.22
The die-off is here: life insurance payouts skyrocket 258% as post-vaxxine deaths rapidly accelerate, 1.2.22
Deaths up 40% among those aged 18-64 based on life insurance claims for 2021 after Covid-19 vaxxine roll outs, 3.1.22
Thank you for your clear and unequivocal answer confirming that you do provide medical cover for side-effects arising from the Covid vaccines. On the one hand, this is reassuring for international civil servants weighing the risk/benefit ratio in making their own decision on whether or not to take a Covid vaccine. On the other hand, there are significant problems involved in reaching any decision for a number of reasons.
First and foremost, the Covid vaccines are novel and experimental and nothing is known about their long-term consequences. Tens of thousands of doctors and epidemiologists around the world have formed large organisations and are issuing alarming warnings about them and indeed, these warnings are borne out by the astronomical numbers of “adverse reactions” and deaths recorded by the various reporting systems, although we are told that these systems historically have recorded only a tiny fraction of the real numbers.
Secondly, the majority of international civil servants are not enrolled in national health insurance schemes and are therefore wholly dependent on private medical insurance. It is vital that our insurers’ actuaries have the right data in advising on the provision of cover so that (a) medical and life insurers remain in business and (b) premiums remain affordable. But how can they do so in the case of a novel and experimental vaccine that has not only never been used on healthy people, let alone whole populations, but has not yet completed its clinical trials? In such an unprecedented actuarial nightmare, insurers could be wiped out entirely.
Thirdly, I would assume that international civil servants are not eligible for any national vaccine compensation schemes, which are typically provided to nationals of countries where international civil servants reside, for the purpose of providing at least a tiny amount of compensation in the event of vaccine injury, given that national governments have granted pharmaceutical companies immunity from lawsuits arising from vaccine injuries. Do you know if the UN, for example, has established such a compensation scheme for its employees?
Fourthly, any international civil servant taking the vaccine and made chronically sick or permanently disabled, as hundreds of thousands of people already have been, despite benefiting from private medical insurance for side-effects, would still have to pay 20% of all claims. In addition, we have to consider that many international civil servants have short-term and very insecure employment contracts so, in the event of chronic illness, they would be highly likely to lose their jobs – without compensation – and be left unemployable and unsupported and having to immediately leave the foreign country in which they had hitherto worked. In either of these cases, they would be left destitute.
Fifthly, since unexposed populations typically already enjoy very high levels of immunity from coronaviruses, including SARS-COV-2, and risk pathogenic priming or antibody-dependent enhancement from taking the vaccine, which means that encountering a real virus after vaccination could provoke a cytokine storm and death, the risk/benefit analysis tends to weigh on the side of caution.
Given all of these major uncertainties, any international civil servant would be well advised to wait before taking any of these vaccines, at least for a couple of years until the Covid vaccine outcomes are clearer.
Covid vaccines are experimental treatments and therefore excluded from cover by most insurers
Not only private medical insurer BUPA in the UK, but lawyer Reiner Fuellmich also reports that medical and life insurance companies in several countries have refused to provide insurance for these experimental treatments. A customer of Manuvie in France has also reported having been told by her life assurer that they would not pay out if she took the experimental vaccine.
The Covid vaccines are acknowledged to be experimental treatments granted only temporary “emergency use authorization” in response to Covid-19. The various authorities that license medicines have specifically stated that these vaccines are neither licensed nor approved for marketing. Some jurisdictions have granted “conditional marketing authorisation”, while others have granted “emergency use authorisation”.
The European Union provided a temporary and strictly COVID-19-related derogation from certain provisions of the GMO Directive for clinical trials on COVID-19 vaccines and treatments that contain or consist of genetically modified organisms (GMOs) in July 2020. This document explicitly states that the derogation applies to “investigational medicinal products” in English and “médicaments expérimentaux” (experimental medicines) in the French version.
The European Medicines Agency issued conditional marketing authorisations for the Covid vaccines currently being distributed. For this authorisation, “less comprehensive pharmaceutical and non-clinical data may be accepted”.
The UK Medicines and Healthcare Products Regulatory Agency (MHRA) granted temporary authorisation for Covid‑19 vaccines In December 2020 and January and February 2021, stating: “The emergency authorisation under Regulation 174 is for emergency use and is not a marketing authorisation.”
BUPA, a UK insurer, has stated that it does not provide cover for the Covid vaccines because they are “an experimental treatment”:
"Side effects arising from the COVID-19 vaccine are not covered under our exclusion for: Complications from excluded or restricted conditions/treatment and experimental treatment exclusion".
All US Food and Drug Administration (FDA) authorization letters for Covid-19 vaccines expressly provide that the vaccines are "An investigational vaccine not licensed for any indication" and the FDA has specifically required that: "All promotional material relating to the COVID-19 vaccine clearly and conspicuously ... state that this product has not been approved or licensed by the FDA".*
*Update 11 September 2021: The recent FDA approval of the Pfizer vaccine relates to a future Pfizer product called Comirnaty which is not currently available in the United States. Pfizer must submit the usual clinical trial data and full disclosure on the ingredients of the treatment in order to take advantage of this future approval and will incur full product liability. It has yet to be seen if Pfizer is willing to risk full product liability as it currently benefits from full immunity under the emergency use authorization. Until that happens, some time in 2023 or 2024 after the clinical trials have been completed, anyone taking the Pfizer vaccine in the US will receive the unapproved vaccine under emergency use authorization, without disclosure of the ingredients and with full immunity from injury lawsuits. The burden of any injuries or death will therefore be carried by the recipient of the experimental gene therapy called "vaccine". (See Drs. Mike Yeadon and Meryl Nass explanation: https://asmaa-algarve.org/covid-19/vaccines/dr-mike-yeadon-s-dr-meryl-nass-response-to-the-fda-approval).
The FDA guidance on emergency use authorization (EUA) of medical products requires the FDA to “ensure that recipients are informed … that they have the option to accept or refuse the EUA product.” Under an EUA, vaccines cannot be made mandatory.
Indeed, the Covid vaccines can be neither licensed nor approved for the very reason that they have not undergone phase 3 clinical trials. In fact, the mass rollout of these vaccines to whole populations is itself the clinical trial required for such approval and licensing to be granted, and these clinical trials will not be complete until 2023.
In 2020, the chairman and other members of the Israeli Supreme Helsinki Committee responsible for approving and supervising experiments on people resigned in protest against a decision by the Health Ministry director general to strip the committee of most of its authority and pursue a policy benefiting pharmaceutical companies seeking to conduct clinical trials in Israel. Israel is acting as the world's testing laboratory, as confirmed by numerous officials, as well as Israeli Prime Minister Benjamin Netanyahu. A complaint of crimes against humanity submitted to the International Criminal Court at The Hague stated that the Israeli Ministry of Health had publicly admitted that 41% of police, military, education and medical personnel who had been vaccinated suffered severe and life-threatening side effects.
Israel’s contract with Pfizer for Covid vaccines, which has only partially been made public, is called a “Real-world epidemiological evidence collaboration agreement”. This means that the Israeli government and Pfizer have agreed to administer vaccines to humans and share the results. This is a “clinical trial” so this agreement should more accurately be called “Human clinical trial agreement”. The section entitled “Definitions” explicitly admits that this is a clinical trial, for it identifies this contract as relating to a “Project” (1.7) consisting of “epidemiological data analyses … involving data collected during the [Ministry of Health’s] vaccination program”.
International civil servants are dependent on private medical insurance
If Allianz is the only company that does provide cover while all others do not, we may reasonably expect Allianz to rapidly go out of business as a result of an explosion in claims, which would leave international civil servants with no private medical insurance, which is a matter of considerable concern to any international civil servant since we are not part of any national health service (NHS) scheme and nor are we eligible for inclusion in any national compensation scheme for vaccine injuries. Were we to find ourselves with no medical insurance and suffering any kind of chronic medical condition, we would very quickly find ourselves bankrupted and destitute.
International civil servants are not enrolled in national health service schemes
Perhaps we should include in these discussions all the governments that host UN duty stations across the world – and those of other international organisations – in order to ascertain if they are willing to grant permanent residence and extend unlimited and free-of-charge medical services to all international civil servants resident on their territory should those civil servants suffer serious side-effects from taking the Covid vaccines and find themselves without private medical insurance either because the insurer refuses to insure against experimental treatments or goes bankrupt from attempting to do so.
International civil servants are not eligible for national vaccine compensation schemes
We should also ask host governments if they would include international civil servants in their national compensation schemes since the vaccine manufacturers have been granted immunity from injury lawsuits arising from these experimental vaccinations. The UK, for example, offers a one-off compensation maximum payment of £120,000 (US$ 137,000; subject to deductions should the claimant be in receipt of other government payments). The UK government has passed regulations reducing legal protection for anyone injured by a COVID-19 vaccine approved for emergency use. Someone in the UK injured by a COVID-19 vaccine is less protected than a person injured by other vaccines since “the UK government effectively gave legal immunity to all the companies supplying and all the health workers injecting the vaccine. The immunity also covers the NHS trusts and foundations that employ the health workers.”
This scheme has been subject to criticism as it does not provide adequate compensation to reassure those who act responsibly by informing themselves before consenting to vaccination. Even were an international civil servant to be included in such a national scheme, after he or she had exhausted this tiny sum, which could take a matter of months if 24-hour care were needed, he or she could become destitute, and even homeless since their inability to work would entail having to leave their country of temporary residence, granted only while they were employed by their international organisation.
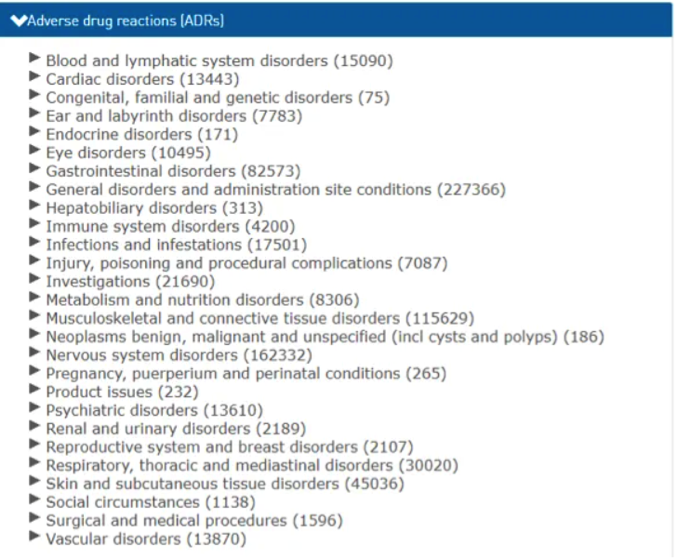
124 pages of Covid vaccine side-effects
The list of side-effects from just one of these vaccines, Pfizer/BioNTech's Comirnaty, recorded by the University of Uppsala in Sweden on their VigiAccess system as of end of March 2021, runs to 124 pages, of which the below is just a sample:
First and foremost, the Covid vaccines are novel and experimental and nothing is known about their long-term consequences. Tens of thousands of doctors and epidemiologists around the world have formed large organisations and are issuing alarming warnings about them and indeed, these warnings are borne out by the astronomical numbers of “adverse reactions” and deaths recorded by the various reporting systems, although we are told that these systems historically have recorded only a tiny fraction of the real numbers.
Secondly, the majority of international civil servants are not enrolled in national health insurance schemes and are therefore wholly dependent on private medical insurance. It is vital that our insurers’ actuaries have the right data in advising on the provision of cover so that (a) medical and life insurers remain in business and (b) premiums remain affordable. But how can they do so in the case of a novel and experimental vaccine that has not only never been used on healthy people, let alone whole populations, but has not yet completed its clinical trials? In such an unprecedented actuarial nightmare, insurers could be wiped out entirely.
Thirdly, I would assume that international civil servants are not eligible for any national vaccine compensation schemes, which are typically provided to nationals of countries where international civil servants reside, for the purpose of providing at least a tiny amount of compensation in the event of vaccine injury, given that national governments have granted pharmaceutical companies immunity from lawsuits arising from vaccine injuries. Do you know if the UN, for example, has established such a compensation scheme for its employees?
Fourthly, any international civil servant taking the vaccine and made chronically sick or permanently disabled, as hundreds of thousands of people already have been, despite benefiting from private medical insurance for side-effects, would still have to pay 20% of all claims. In addition, we have to consider that many international civil servants have short-term and very insecure employment contracts so, in the event of chronic illness, they would be highly likely to lose their jobs – without compensation – and be left unemployable and unsupported and having to immediately leave the foreign country in which they had hitherto worked. In either of these cases, they would be left destitute.
Fifthly, since unexposed populations typically already enjoy very high levels of immunity from coronaviruses, including SARS-COV-2, and risk pathogenic priming or antibody-dependent enhancement from taking the vaccine, which means that encountering a real virus after vaccination could provoke a cytokine storm and death, the risk/benefit analysis tends to weigh on the side of caution.
Given all of these major uncertainties, any international civil servant would be well advised to wait before taking any of these vaccines, at least for a couple of years until the Covid vaccine outcomes are clearer.
Covid vaccines are experimental treatments and therefore excluded from cover by most insurers
Not only private medical insurer BUPA in the UK, but lawyer Reiner Fuellmich also reports that medical and life insurance companies in several countries have refused to provide insurance for these experimental treatments. A customer of Manuvie in France has also reported having been told by her life assurer that they would not pay out if she took the experimental vaccine.
The Covid vaccines are acknowledged to be experimental treatments granted only temporary “emergency use authorization” in response to Covid-19. The various authorities that license medicines have specifically stated that these vaccines are neither licensed nor approved for marketing. Some jurisdictions have granted “conditional marketing authorisation”, while others have granted “emergency use authorisation”.
The European Union provided a temporary and strictly COVID-19-related derogation from certain provisions of the GMO Directive for clinical trials on COVID-19 vaccines and treatments that contain or consist of genetically modified organisms (GMOs) in July 2020. This document explicitly states that the derogation applies to “investigational medicinal products” in English and “médicaments expérimentaux” (experimental medicines) in the French version.
The European Medicines Agency issued conditional marketing authorisations for the Covid vaccines currently being distributed. For this authorisation, “less comprehensive pharmaceutical and non-clinical data may be accepted”.
The UK Medicines and Healthcare Products Regulatory Agency (MHRA) granted temporary authorisation for Covid‑19 vaccines In December 2020 and January and February 2021, stating: “The emergency authorisation under Regulation 174 is for emergency use and is not a marketing authorisation.”
BUPA, a UK insurer, has stated that it does not provide cover for the Covid vaccines because they are “an experimental treatment”:
"Side effects arising from the COVID-19 vaccine are not covered under our exclusion for: Complications from excluded or restricted conditions/treatment and experimental treatment exclusion".
All US Food and Drug Administration (FDA) authorization letters for Covid-19 vaccines expressly provide that the vaccines are "An investigational vaccine not licensed for any indication" and the FDA has specifically required that: "All promotional material relating to the COVID-19 vaccine clearly and conspicuously ... state that this product has not been approved or licensed by the FDA".*
*Update 11 September 2021: The recent FDA approval of the Pfizer vaccine relates to a future Pfizer product called Comirnaty which is not currently available in the United States. Pfizer must submit the usual clinical trial data and full disclosure on the ingredients of the treatment in order to take advantage of this future approval and will incur full product liability. It has yet to be seen if Pfizer is willing to risk full product liability as it currently benefits from full immunity under the emergency use authorization. Until that happens, some time in 2023 or 2024 after the clinical trials have been completed, anyone taking the Pfizer vaccine in the US will receive the unapproved vaccine under emergency use authorization, without disclosure of the ingredients and with full immunity from injury lawsuits. The burden of any injuries or death will therefore be carried by the recipient of the experimental gene therapy called "vaccine". (See Drs. Mike Yeadon and Meryl Nass explanation: https://asmaa-algarve.org/covid-19/vaccines/dr-mike-yeadon-s-dr-meryl-nass-response-to-the-fda-approval).
The FDA guidance on emergency use authorization (EUA) of medical products requires the FDA to “ensure that recipients are informed … that they have the option to accept or refuse the EUA product.” Under an EUA, vaccines cannot be made mandatory.
Indeed, the Covid vaccines can be neither licensed nor approved for the very reason that they have not undergone phase 3 clinical trials. In fact, the mass rollout of these vaccines to whole populations is itself the clinical trial required for such approval and licensing to be granted, and these clinical trials will not be complete until 2023.
In 2020, the chairman and other members of the Israeli Supreme Helsinki Committee responsible for approving and supervising experiments on people resigned in protest against a decision by the Health Ministry director general to strip the committee of most of its authority and pursue a policy benefiting pharmaceutical companies seeking to conduct clinical trials in Israel. Israel is acting as the world's testing laboratory, as confirmed by numerous officials, as well as Israeli Prime Minister Benjamin Netanyahu. A complaint of crimes against humanity submitted to the International Criminal Court at The Hague stated that the Israeli Ministry of Health had publicly admitted that 41% of police, military, education and medical personnel who had been vaccinated suffered severe and life-threatening side effects.
Israel’s contract with Pfizer for Covid vaccines, which has only partially been made public, is called a “Real-world epidemiological evidence collaboration agreement”. This means that the Israeli government and Pfizer have agreed to administer vaccines to humans and share the results. This is a “clinical trial” so this agreement should more accurately be called “Human clinical trial agreement”. The section entitled “Definitions” explicitly admits that this is a clinical trial, for it identifies this contract as relating to a “Project” (1.7) consisting of “epidemiological data analyses … involving data collected during the [Ministry of Health’s] vaccination program”.
International civil servants are dependent on private medical insurance
If Allianz is the only company that does provide cover while all others do not, we may reasonably expect Allianz to rapidly go out of business as a result of an explosion in claims, which would leave international civil servants with no private medical insurance, which is a matter of considerable concern to any international civil servant since we are not part of any national health service (NHS) scheme and nor are we eligible for inclusion in any national compensation scheme for vaccine injuries. Were we to find ourselves with no medical insurance and suffering any kind of chronic medical condition, we would very quickly find ourselves bankrupted and destitute.
International civil servants are not enrolled in national health service schemes
Perhaps we should include in these discussions all the governments that host UN duty stations across the world – and those of other international organisations – in order to ascertain if they are willing to grant permanent residence and extend unlimited and free-of-charge medical services to all international civil servants resident on their territory should those civil servants suffer serious side-effects from taking the Covid vaccines and find themselves without private medical insurance either because the insurer refuses to insure against experimental treatments or goes bankrupt from attempting to do so.
International civil servants are not eligible for national vaccine compensation schemes
We should also ask host governments if they would include international civil servants in their national compensation schemes since the vaccine manufacturers have been granted immunity from injury lawsuits arising from these experimental vaccinations. The UK, for example, offers a one-off compensation maximum payment of £120,000 (US$ 137,000; subject to deductions should the claimant be in receipt of other government payments). The UK government has passed regulations reducing legal protection for anyone injured by a COVID-19 vaccine approved for emergency use. Someone in the UK injured by a COVID-19 vaccine is less protected than a person injured by other vaccines since “the UK government effectively gave legal immunity to all the companies supplying and all the health workers injecting the vaccine. The immunity also covers the NHS trusts and foundations that employ the health workers.”
This scheme has been subject to criticism as it does not provide adequate compensation to reassure those who act responsibly by informing themselves before consenting to vaccination. Even were an international civil servant to be included in such a national scheme, after he or she had exhausted this tiny sum, which could take a matter of months if 24-hour care were needed, he or she could become destitute, and even homeless since their inability to work would entail having to leave their country of temporary residence, granted only while they were employed by their international organisation.
124 pages of Covid vaccine side-effects
The list of side-effects from just one of these vaccines, Pfizer/BioNTech's Comirnaty, recorded by the University of Uppsala in Sweden on their VigiAccess system as of end of March 2021, runs to 124 pages, of which the below is just a sample:
Tens of thousands of vaccine deaths and hundreds of thousands of adverse effects
It is further reported with regard to the US Centers for Disease Control (CDC) Vaccine Adverse Event Reporting System (VAERS), that it is recognised in scientific circles that this voluntary reporting system is historically unreliable as an indicator of the true level of adverse reactions because typically only 1% of those suffering adverse events or those providing medical care actually report them. We may therefore suppose the true number of adverse events to be astronomically higher. See the following extract from a report submitted to the US Agency for Healthcare Research and Quality at the US Department of Health and Human Services in 2010:
Fewer than 1% of vaccine adverse events are reported. Low reporting rates preclude or slow the identification of “problem” drugs and vaccines that endanger public health. New surveillance methods for drug and vaccine adverse effects are needed.
In October 2020, the US Food and Drug Administration published the following extensive list of the adverse reactions they expected to see as a result of the use of these vaccines (and emphasized that this was only a partial list), as follows:
And indeed we find confirmation of exactly these side-effects, along with numerous others, reported by the various reporting systems in different countries, the 584 cases of Bell’s Palsy or similar reported in the US being just one example. The Medical Director of a hospital in the United Kingdom has said that the “levels of sickness after vaccination is unprecedented” among NHS staff, confirming that some are even suffering neurological symptoms, which is having a “huge impact on the health service functioning”.
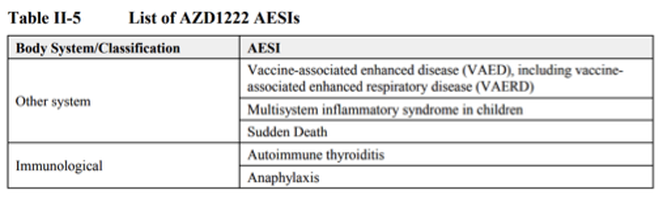
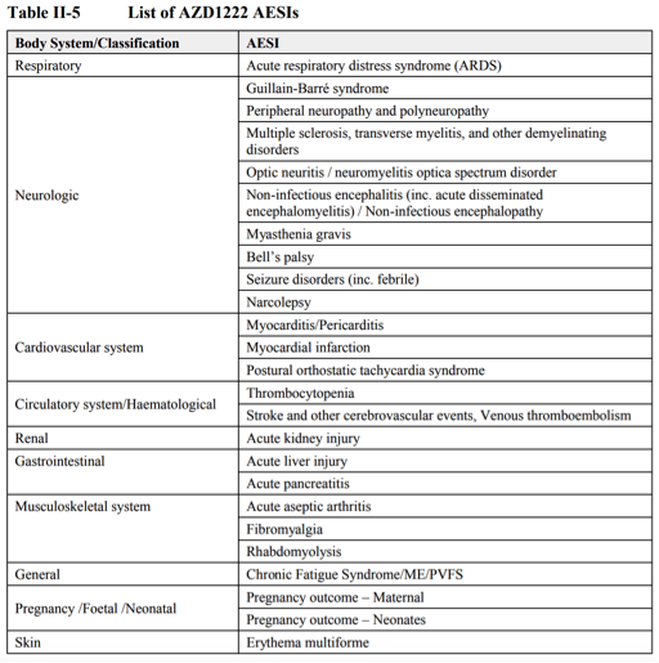
The list of Adverse Events of Special Interest (AESIs) applicable to the AstraZeneca vaccine that had been established from research and clinical trials by 4 November, 2020, 3 months before it was authorised for temporary use, include the following:
It is further reported with regard to the US Centers for Disease Control (CDC) Vaccine Adverse Event Reporting System (VAERS), that it is recognised in scientific circles that this voluntary reporting system is historically unreliable as an indicator of the true level of adverse reactions because typically only 1% of those suffering adverse events or those providing medical care actually report them. We may therefore suppose the true number of adverse events to be astronomically higher. See the following extract from a report submitted to the US Agency for Healthcare Research and Quality at the US Department of Health and Human Services in 2010:
Fewer than 1% of vaccine adverse events are reported. Low reporting rates preclude or slow the identification of “problem” drugs and vaccines that endanger public health. New surveillance methods for drug and vaccine adverse effects are needed.
In October 2020, the US Food and Drug Administration published the following extensive list of the adverse reactions they expected to see as a result of the use of these vaccines (and emphasized that this was only a partial list), as follows:
- Acute disseminated encephalomyelitis
- Acute myocardial infarction
- Anaphylaxis
- Arthritis and arthralgia/joint pain
- Autoimmune disease
- Convulsions/seizures
- Deaths
- Disseminated intravascular coagulation
- Encephalitis/myelitis/encephalomyelitis/meningoencephalitis/meningitis/ encephalopathy
- Guillain-Barré syndrome
- Kawasaki disease
- Multisystem Inflammatory Syndrome in Children
- Myocarditis/pericarditis
- Narcolepsy and cataplexy
- Non-anaphylactic allergic reactions
- Other acute demyelinating diseases
- Pregnancy and birth outcomes
- Stroke
- Thrombocytopenia
- Transverse myelitis
- Vaccine enhanced disease adverse effects
- Venous thromboembolism
And indeed we find confirmation of exactly these side-effects, along with numerous others, reported by the various reporting systems in different countries, the 584 cases of Bell’s Palsy or similar reported in the US being just one example. The Medical Director of a hospital in the United Kingdom has said that the “levels of sickness after vaccination is unprecedented” among NHS staff, confirming that some are even suffering neurological symptoms, which is having a “huge impact on the health service functioning”.
The list of Adverse Events of Special Interest (AESIs) applicable to the AstraZeneca vaccine that had been established from research and clinical trials by 4 November, 2020, 3 months before it was authorised for temporary use, include the following:
In the US, there have been more deaths related to vaccines in 2021 in less than 3 months than there were during the entire previous decade. On 26 March 2021, the CDC's VAERS system reported that, following COVID vaccines, between 14 December 2020 and 19 March 2021, there had been 44,606 reports of adverse events, including 2,050 deaths and 7,095 serious injuries. If indeed this represents only 1% of the true count, then these figures could indicate an actual US rate of 205,000 deaths, 4.5 million adverse events and 709,500 serious injuries.
In the UK, as of 21 February 2021, the Medicines and Healthcare products Regulatory Agency (MHRA) had received 42,917 reports of 157,637 suspected adverse reactions (ADRs). 244 of these were of the participant dying shortly after vaccination. Of these ADRs, 1,516 were cardiac disorders (of which 30 were fatal); 17,597 gastrointestinal disorders (5 fatal); 3,016 infectious disorders (32 fatal); 2,057 metabolic disorders (2 fatal); 34,656 disorders of the nervous system (14 fatal); 4,059 respiratory disorders (10 fatal), and 56,377 general disorders (146 fatal). In total, including the BioNTech/Pfizer vaccine, out of 18.4 million doses administered in the UK, there have been 242,651 ADRs resulting in 460 deaths occurring shortly after vaccination.
As of 21 March 2021, a total of 700 deaths were reported in the UK since the start of vaccination in mid-December 2020.
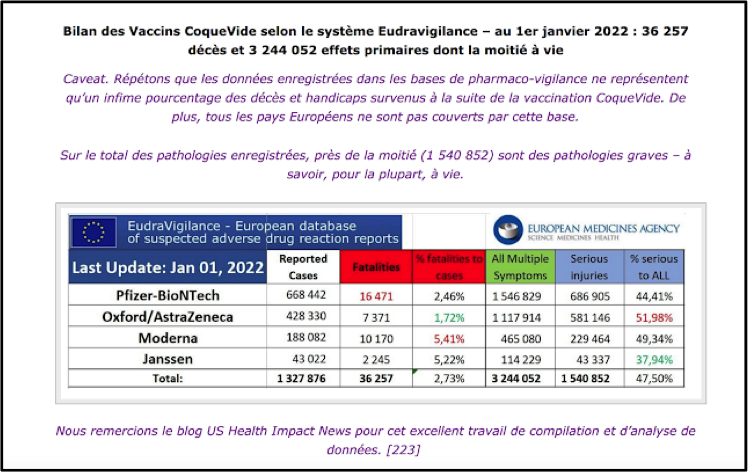
EUDRAVIGILANCE, the European database of suspected adverse drug reaction reports, reported 5,980 deaths and 272,044 injuries as of 3 April 2021, involving the Pfizer/BioNTech, Moderna and AstraZeneca vaccines. Here is a graphic showing the unprecedented spike in adverse reactions in the EMA area since the start of administration of the Covid vaccines:
In the UK, as of 21 February 2021, the Medicines and Healthcare products Regulatory Agency (MHRA) had received 42,917 reports of 157,637 suspected adverse reactions (ADRs). 244 of these were of the participant dying shortly after vaccination. Of these ADRs, 1,516 were cardiac disorders (of which 30 were fatal); 17,597 gastrointestinal disorders (5 fatal); 3,016 infectious disorders (32 fatal); 2,057 metabolic disorders (2 fatal); 34,656 disorders of the nervous system (14 fatal); 4,059 respiratory disorders (10 fatal), and 56,377 general disorders (146 fatal). In total, including the BioNTech/Pfizer vaccine, out of 18.4 million doses administered in the UK, there have been 242,651 ADRs resulting in 460 deaths occurring shortly after vaccination.
As of 21 March 2021, a total of 700 deaths were reported in the UK since the start of vaccination in mid-December 2020.
EUDRAVIGILANCE, the European database of suspected adverse drug reaction reports, reported 5,980 deaths and 272,044 injuries as of 3 April 2021, involving the Pfizer/BioNTech, Moderna and AstraZeneca vaccines. Here is a graphic showing the unprecedented spike in adverse reactions in the EMA area since the start of administration of the Covid vaccines:
Having had had only 16 Covid deaths prior to starting Covid-19 vaccinations, the tiny island of Gibraltar had 53 dead within 10 days.
With regard to Israel, a study emanating from the Faculty of Medicine Emerging Infectious and Tropical Diseases at Aix-Marseille University has revealed that Pfizer's vaccine killed “about 40 times more (elderly) people” (200 of 100,000 as compared to 4.91) and “260 times more of the young” (50 of 100,000 compared to 0.19) than “what the COVID-19 virus would have claimed in the given time frame”.
Conclusion
I note that the small print of your email states that “AWP Health & Life SA, acting through its Irish Branch, is a limited company with a capital of €65,190,446”. This is a very small sum in terms of the potential for a huge burden of medical costs that could arise from your clients’ take-up of the Covid experimental vaccines.
In that light, Allianz’s decision to cover any side-effects arising from the vaccines is concerning. In the event of Allianz being unable to continue operating, international civil servants who rely on their private medical insurance, since they are not part of any national health insurance schemes, could be left with no medical cover at all. In any circumstances this is concerning; in the present situation with great medical uncertainty for everyone, this is alarming.
Another concern is that international civil servants are presumably in the same situation as everyone else, unable to bring an injury lawsuit against a pharmaceutical company in the event of injury from these experimental vaccines. However, unlike everyone else, they cannot benefit from any national compensation scheme. That being the case, the UN itself should be providing an adequate compensation scheme if the UN chooses to offer the vaccines to its staff worldwide.
In the light of these concerns, an international civil servant may consider that accepting the risk of contracting Covid, for which the survival rate for those aged 20-49 is 99.98% and for those aged 50-69 is 99.5%, is preferable to the risk of taking the experimental vaccine given, for example, that 0.8% of vaccinated people experienced adverse reactions at a US mass-vaccination site in early April 2021, which may be more representative of the true number of people being injured by these experimental vaccines.
Best regards
Claire Edwards
UN Office at Vienna (Retired)
With regard to Israel, a study emanating from the Faculty of Medicine Emerging Infectious and Tropical Diseases at Aix-Marseille University has revealed that Pfizer's vaccine killed “about 40 times more (elderly) people” (200 of 100,000 as compared to 4.91) and “260 times more of the young” (50 of 100,000 compared to 0.19) than “what the COVID-19 virus would have claimed in the given time frame”.
Conclusion
I note that the small print of your email states that “AWP Health & Life SA, acting through its Irish Branch, is a limited company with a capital of €65,190,446”. This is a very small sum in terms of the potential for a huge burden of medical costs that could arise from your clients’ take-up of the Covid experimental vaccines.
In that light, Allianz’s decision to cover any side-effects arising from the vaccines is concerning. In the event of Allianz being unable to continue operating, international civil servants who rely on their private medical insurance, since they are not part of any national health insurance schemes, could be left with no medical cover at all. In any circumstances this is concerning; in the present situation with great medical uncertainty for everyone, this is alarming.
Another concern is that international civil servants are presumably in the same situation as everyone else, unable to bring an injury lawsuit against a pharmaceutical company in the event of injury from these experimental vaccines. However, unlike everyone else, they cannot benefit from any national compensation scheme. That being the case, the UN itself should be providing an adequate compensation scheme if the UN chooses to offer the vaccines to its staff worldwide.
In the light of these concerns, an international civil servant may consider that accepting the risk of contracting Covid, for which the survival rate for those aged 20-49 is 99.98% and for those aged 50-69 is 99.5%, is preferable to the risk of taking the experimental vaccine given, for example, that 0.8% of vaccinated people experienced adverse reactions at a US mass-vaccination site in early April 2021, which may be more representative of the true number of people being injured by these experimental vaccines.
Best regards
Claire Edwards
UN Office at Vienna (Retired)